Objective: To compare the Vacuum-Assisted Closure technique to the conventional wound management in the treatment of patients with deep sternal wound infection after cardiac surgery.
Methods: A total of 4400 patients underwent open heart surgery at Queen Alia Heart Institute between July 2001 and July 2005.Thirty six patients developed poststernotomy deep sternal wound infections (0.8%).These patients were treated by two different modalities. Twenty patients (group I) were treated by the conventional wound management. The other (group II) 16 patients were treated by Vacuum Assisted Closure Technique. The two groups were comparable with regards to age, sex, weight, associated diseases, presenting postoperative day, infecting organism, and risk factors for deep sternal wound infection.
Results: Patients treated by Vacuum Assisted Closure (group II) had a lower mean duration treatment time 10.5 days while the mean duration treatment time was 32 days in group I .Mean hospital duration stay was 17.6 days in group II, however it was 40 days in group I. Mean long term follow up period of both groups was six (range 2-14) months.Re-admissions and repeated surgical procedures was 30% in group I and 12.5% in group II. Perioperative mortality was higher in group I (10%) than in group II (6.25%).
Conclusion: The Vacuum-Assisted Closure Technique for deep sternal wound infection management has many advantages over conventional methods. Vacuum Assisted Closure offers the benefits of an optimal physiological environment of closed technique and the efficient removal of necrotic debris seen with the open technique. Moreover, Vacuum Assisted Closure shortened wound healing and hospital stay, cost-effective, and safe.
Key words: Deep sternal wound infection, Vacuum assisted closure, Sternotomy
JRMS August 2007; 14(2): 31-37
Introduction
Deep sternal wound infection (DSWI) is a serious and potentially lethal complication of cardiac surgery with both human and economic consequences.(1,2) The reported incidence varies from 1-5% of all midsternotomy procedures.(3,4) Sternotomy infections following cardiac surgery may involve superficial wounds, sternum osteomyelitis and mediastinitis. DSWI remains a major cause of morbidity, resulting in prolonged hospital stay, repeated surgical procedures, and increased perioperative mortality. Associated mortality has been reported to range between 10-47% in different studies.(5,6)
Despite many recent advances in the understanding of the physiological basics of wound healing, their treatment remains a difficult challenge. Various management strategies have been used to treat such cases. The majority of cardiac surgeons used to manage DSWI with frequent debridement, dressing, rewiring and closed drainage with or without antiseptic solution irrigation (the conventional approach).
Others used aggressive debridement with rotational muscle flaps.(7) During the last decade Vacuum Assisted Closure (VAC) has been introduced as an alternative method of wound treatment. It is based on applying sub atmospheric pressure to the wound through a polyurethane foam dressing and has been shown to improve wound healing and reduce bacterial colonization.
In this study we report our experience in the treatment of DSWI with VAC in comparison to conventional wound management.
Methods
A total of 4400 patients underwent open heart surgery at Queen Alia Heart Institute between July 2001 and July 2005. Thirty six patients developed poststernotomy deep sternal wound infections (0.8%).
Table I and II shows the different patient demographics, EuroScore,surgical procedures, infecting organism, risk factors for deep sternal wound infection (Diabetes mellitus, body mass index ≥ 30 kg/m2,chronic obstructive pulmonary disease, renal failure, low ejection fraction of the left ventricle, emergency surgery), hospital stay, treatment time, and time of presentation.
Table I: Demographic characteristics and relevant variables among the study groups
|
Variable
|
VAC* Therapy
|
Conventional
Treatment
|
|
N
|
%
|
|
|
|
Number of patients
|
16
|
|
20
|
|
|
Gender:
|
|
|
|
|
|
Male
|
6
|
37.5
|
12
|
60
|
|
Female
|
10
|
62.5
|
8
|
40
|
|
Surgical procedure:
|
|
|
|
|
|
CABG**
|
11
|
68.7
|
15
|
75
|
|
Other procedures
|
5
|
31.3
|
5
|
25
|
|
Diabetes mellitus
|
10
|
62.5
|
6
|
30
|
|
Obesity (BMI***
≥ 30 kg/m2)
|
4
|
25
|
6
|
30
|
|
LVEF**** < 0.30
|
8
|
50
|
8
|
40
|
|
COPD*****
|
5
|
20
|
9
|
45
|
|
Emergency surgery
|
2
|
12.5
|
3
|
15
|
|
Renal failure
|
1
|
6.25
|
2
|
10
|
|
Infecting micro-organism:
Staphylococcus aureus
Staphylococcus epidermidis
Klebsiella
Pseudomonas
Escherichia coli
No growth
|
6
5
0
4
1
0
|
37.5
31.25
0
25
6.25
0
|
7
6
2
4
0
1
|
35
30
10
20
0
5
|
|
|
Mean
|
Range
|
Mean
|
Range
|
|
Age (years)
|
67.6
|
55-75
|
68
|
52-87
|
|
Euro SCORE
|
7.8
|
4-11
|
7.2
|
3-10
|
* VAC: Vacuum assisted closure ** CABG: Coronary artery bypass grafting *** BMI: Body mass index **** LVEF: Left ventricular ejection fraction ***** COPD: Chronic obstructive airway disease
Table II: Detailed demographic characteristics and procedure information of group II (VAC) patients
|
No.
|
Age
(year)
|
gender
|
primary procedure
|
No. of debridement
|
Presentation
After surgery ( days)
|
Final closure
|
Duration of VAC
|
Hospitalization
(days)
|
|
1.
|
70
|
F
|
CABG
|
1
|
8
|
DSC
|
7
|
16
|
|
2.
|
65
|
M
|
CABG
|
2
|
10
|
DSC
|
10
|
20
|
|
3.
|
62
|
F
|
CABG+MVR
|
2
|
7
|
DSC
|
9
|
16
|
|
4.
|
71
|
M
|
CABG
|
-
|
10
|
DSC
|
7
|
17
|
|
5.
|
75
|
M
|
AVR
|
2
|
7
|
BPF
|
17
|
25
|
|
6.
|
65
|
F
|
CABG
|
-
|
9
|
BPF
|
7
|
14
|
|
7.
|
55
|
F
|
MVR
|
2
|
8
|
DSC
|
12
|
10
|
|
8.
|
70
|
F
|
CABG
|
3
|
12
|
BPF
|
12
|
30 (Died)
|
|
9.
|
69
|
F
|
CABG
|
2
|
10
|
DSC
|
8
|
20
|
|
10.
|
65
|
F
|
CABG
|
2
|
10
|
DSC
|
9
|
17
|
|
11.
|
72
|
M
|
CABG+MVR
|
1
|
9
|
BPF
|
13
|
16
|
|
12.
|
69
|
M
|
CABG
|
2
|
15
|
DSC
|
20
|
16
|
|
13.
|
70
|
F
|
CABG
|
3
|
12
|
DSC
|
14
|
25
|
|
14.
|
62
|
F
|
CABG
|
2
|
14
|
DSC
|
12
|
30
|
|
15.
|
68
|
M
|
CABG+AVR
|
1
|
12
|
DSC
|
7
|
17
|
|
16.
|
73
|
F
|
CABG
|
2
|
13
|
DSC
|
12
|
18
|
VAC: Vacuum assisted closure, CABG: Coronary artery bypass grafting, AVR: Aortic valve replacement, MVR: Mitral valve replacement, DSC: Delayed secondary closure, BPF: Bilateral pectoral flap.
In July 2003, VAC was introduced to our department. Until then, all patients were treated conventionally (group I). Thereafter, all cases of DSWI were treated using VAC (group II) which was compared retrospectively to group I.
In group I, treatment consisted of reexploration, removal of sternal wiring, debridement of all nonviable tissues, washing the wound with hydrogen peroxide and rinsing it with saline solution. The extent of infection and debridement determined the subsequent treatment options.
Patients with infection confined to the sternal wound edges where debridement revealed underlying healthy bone and varying degrees of infection of pre-sternal tissues were treated by rewiring of the sternum and conventional open treatment of the pre-sternal tissues in group I (n=14) and by vacuum-assisted suction drainage in group II (n=11). Six / 20 patients in group I had DSWI with evidence of infection in the retrosternal space and extensive infection of the presternal tissues, necessitating wide debridement of the subcutaneous tissues but allowing for rewiring of the sternum were treated by placement of two 16 Charriere catheters for irrigation and two 28 Charriere chest tubes for drainage of the mediastinum, sternal rewiring and conventional open treatment of the presternal tissues.
Irrigation with saline solution was continued until drainage was clear and chest tubes then removed. In group II, five of such patients were treated successfully by VAC.
In group I, 4/20 patients and 3/16 patient in group II with DSWI with extensive involvevement of the mediastinum and sternum required resection of the whole sternum and had subsequent pectoralis major advancement flap procedures.
All 16 patients in group II received VAC as a first-line treatment (Fig. 1). After reexploration, removal of sternal wires and debridement of all infected and nonviable tissues, the wounds were washed with hydrogen peroxide, rinsed with saline solution and the sternum rewired.
Then polyurethane foam with an embedded noncollapsible evacuation tube was tailored to fit the size of the tissue defect. In deep wounds several foam layers were placed on top of each other. The whole area was then covered with a transparent adhesive drape. The foam was connected through the evacuation tube with a VAC vacuum pump (KCI International, San Antonio, Texas).
The suction generated a continuous vacuum in the polyurethane foam, producing a high contact zone in the wound–foam interface, and thus a vacuum seal was achieved. The VAC pump was set to generate a negative pressure of 75-125 mmHg. Secretions were drained into an exchangeable reservoir, connected to the VAC pump. The vacuum caused the foam to reduce in volume, pulling the wound edges together and giving it stability (Fig. 2).
The foam dressing and evacuation tube were changed every 2–3 days, to prevent granulation tissue ingrowth into the foam (Fig. 3, 4). These procedures were carried out on the ward, as they are painless and do not require analgesia. Upon change of dressing the wounds were regularly inspected and debrided as necessary. After granulation tissue filled the defect and all microbiological cultures were negative, delayed secondary closure of the wound was performed.
Perioperatively patients in both groups routinely received antibiotic prophylaxis with 1gm intravenous Ceftriaxone twice daily and 500 mg intravenous Amikacin three times daily for 48 hours. Once DSWI was diagnosed, broad-spectrum antibiotic treatment was initiated and modified according to culture results. Patients were discharged once their wounds were clean and if their clinical condition was satisfactory.
All patients with superficial wound infection and stable sternum were excluded from the study.
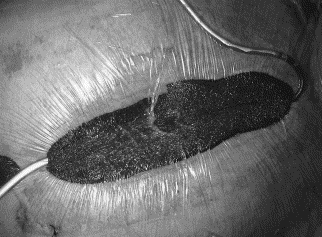
Fig. 1: The vacuum-assisted wound closure system in situ with 100 mm Hg of suction
Through the use of two sponges, the suction is spread equally over the wound and the sternal parts are better stabilized (19).
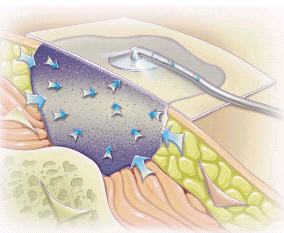 Fig. 2:
Fig. 2: A diagram showing the dressing in situ where the negative pressure approximates dehiscent tissues together with absorption of tissue fluids.(20)
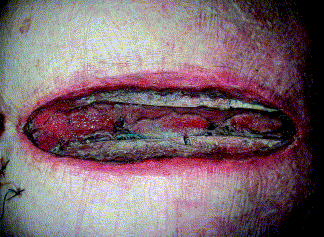 Fig. 3:
Fig. 3: A patient after aortic valve replacement and coronary artery bypass grafting on postoperative day 10 in whom deep sternal infection was detected on postoperative day 7. Shown is the wound situation after the first 72 hours with the vacuum-assisted wound closure system. Necrotic tissue is seen between the sternal parts. First signs of the promotion of granulation tissue proliferation can also be seen, which gives the wound the "beefy red" color (18)
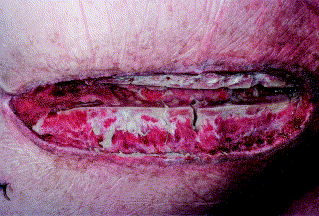
Fig. 4: The same patient in Figure 3 on postoperative day 17 and after the third vacuum-assisted wound closure dressing change. The wound is completely oversewn with viable granulation tissue, which gives the wound a paler color, as the amount of collagen has increased. Furthermore, the volume of the wound defect is decreasing. Signs of infection have completely resolved. In this patient, a pectoralis muscle flap closure was subsequently performed (18)
Results
A. Conventional group
There were 20 patients in the conventional group, aged 52–87 years (mean 68 years). Gender distribution in this group was 12 males and 8 females. Fifteen patients had isolated coronary artery bypass grafting (CABG), two underwent CABG and aortic valve replacement (AVR), one had CABG and mitral valve repair, one had isolated AVR, and one had AVR and mitral valve replacement (MVR). In 14 of these patients left internal mammary artery was used as bypass conduit. None has bilateral internal mammary arteries harvested.
Eighteen/20 patients (90%) had one or more risk factor for DSWI: Six patients were diabetic, nine had chronic obstructive pulmonary disease (COPD), two had preoperative renal failure, and six were overweight (body mass index≥30 kg/m2) (Table I). All but one patient had positive microbiology cultures from their sternal wound specimens including Staphylococcus aureus (n=7), Staphylococcus epidermidis (n=6), Klebsiella (n=2), and Pseudomonas (n=4).
The time of presentation with DSWI ranged from 5th -14th (mean: 7th) postoperative day. The duration of treatment lasted a mean of 32(range 15-62) days and total hospital stay was a mean of 40(range 18-70) days. Early perioperative mortality in this group was 2/20 patients (10%).
Six patients required closed chest irrigation drainage and four patients underwent secondary pectoralis major muscle advancement flap procedures.
B. Vacuum-assisted group
There were 16 patients in the VAC group, aged 55–75 years (mean 67.6 years). Gender distribution in this group was 6 males and 10 females. Eleven patients had isolated CABG, two underwent combined CABG and MVR, one had combined CABG and AVR, one had isolated AVR, and one had isolated MVR.
In 10 of these patients left internal mammary artery was used as a bypass conduit. None had bilateral mammary artery harvested. All patients had one or more risk factor for DSWI. Ten patients were diabetics, five had COPD, one had preoperative renal failure, and four were overweight. All patients had positive microbiology from their sternal wound cultures including Staphylococcus aureus (n=6), Staphylococcus epidermidis (n=5), Pseudomonas (n=4), and Escherichia coli (n=1).
The time of presentation with DSWI varied from postoperative day 7 to 15 (mean 10.5 days). The duration of vacuum assisted closure treatment lasted a mean of 11 (range 7-20 days). The length of hospital stay ranged from 10 to 30 (mean 17.6) days. Hospital mortality was 6.25% in this group.
When comparing both treatment groups (Table III); we found that the perioperative mortality was higher in the conventional group (group I) 10% (n=2) vs. 6.25 %( n=1) in VAC group (group II) which is statically not significant (P< 0.1), however, the duration of treatment was longer in group I than group II (P<0.01), and the total length of hospital stay was also increased in group I (P<0.05). The formation of granulation tissue that ultimately lead to secondary wound healing and the reduction in wound size was grossly faster in the VAC group than in the conventional group (Fig. 2).
Table III: Comparison of duration of treatment, hospital stay, readmissions, and outcome in both groups
|
|
VAC therapy
|
Conventional
treatment
|
P-value
|
|
Treatment
duration(days)
|
10.5(5-17)
|
32(15-62)
|
<0.01
|
|
Total hospital (days)
|
17.6(10-30)
|
40(18-70)
|
<0.05
|
|
Re-admission &
repeated procedure (%)
|
12.5
|
30
|
<0.05
|
|
Perioperative
mortality (%)
|
6.25
|
10
|
<0.1
|
VAC: Vacuum Assisted Closure
DiscussionDeep sternal wound infection (DSWI) is a rare but potentially life threatening complication of cardiac surgery.(1) Its incidence of about 1-5% has remained unchanged although profound efforts have been directed towards identifying and eliminating its causes and risk factors.
For the affected patient the consequences are substantial as the morbidity is considerable and the associated mortality of 10–50% is high.(2,3) In our series of patients, the incidence of DSWI was 0.8% over the past 4 years. This rate was constant over the last 2 years, although strict prophylactic measures were implicated. The overall DSWI associated mortality in the two groups of patients was 8.3 %( 10% in group I and 6.25 in group II). These findings are comparable to those of other study groups.(8-10)
The conventional treatment (group I) of DSWI and mediastinitis consisted of reexploration and assessment of the extent of infection, followed by complete excision of all nonviable and infected tissues. Thereafter, depending on the extent of debridement, delayed secondary closure was attempted including the use of closed irrigation systems.
These are often cumbersome in use and have numerous problems like absorption of irrigation solution, blockage, hazardous measurement and culture of exudate as well as no access for repeated debridement. Similar experiences with closed irrigation systems are described in the current literature.(11) In patients with deep-seated infection, extensive wound debridement and open drainage became necessary.
According to our experience, however, this bears a number of problems, in particular the disadvantage of chest instability necessitating mechanical ventilation and the heavy burden on nursing staff. Alternatively muscle flap closure of the defect could be attempted, with its advantages of increasing wound vascularity and providing some stability to the chest.(7,11) However, implanting a muscle flap into a poorly vascularized and potentially infected surrounding in an acutely ill patient has a considerable rate of short-term failure and a number of long term functional deficits.(12)
The VAC technique was first introduced by Argenta and colleagues in the field of plastic surgery for the treatment of pressure ulcers and chronic wounds. They stated that theoretically, the VAC have several advantages over open packing for deep sternal wounds, the uniform negative pressure, when applied to a wound, permits arteriolar dilatation that, in effect, promotes granulation tissue proliferation(13).
It combines the benefits of both closed and open wound treatment(14-16). Since then, the VAC has been shown to have many applications in wound care .Vacuum sealing protects the wound against contamination and furthermore, excess interstitial and putrid wound secretions are continuously removed thereby preventing fluid retention. The vacuum suction stabilizes the wound, avoids wound edge retraction and reduces pain. Patients can be mobilized early and even discharged earlier.
In our opinion, the foremost advantages of VAC over conventional therapy are the accelerated formation of granulation tissue, a marked improvement in wound vascularization and decreased bacterial colonialization. The stimulation of tissue granulation makes secondary closure with myocutaneous flaps unnecessary, as even large defects are covered within a short period of time. Therefore, additional major surgical procedures can be avoided.
In conventional treatment, wound dressings have to be changed 3-4 times daily, whereas in VAC the polyurethane foam only needs changing every 2–3 days.
It is therefore less discomforting and time-consuming for patients and nursing staff. Several other authors also have reported their experiences with VAC for the treatment of poststernotomy DSWI.(17-19) Sjögren et al.(17) stated that properly applied VAC therapy is a safe and reliable option in poststernotomy mediastinitis, with excellent survival and very low failure rate compared with conventional treatments. Gustafson et al.(18) pointed out that VAC therapy is a safe and reproducible option to bridge patients with postoperative DSWI to complete healing.
Reconstruction of the sternum was achieved in all patients without the use of muscle or omental flap surgery. Fleck et al.(19) suggested that the VAC system is a valuable and effective adjunct to conventional and established treatment modalities in the management of patients with sustained sternal wound infection after
cardiac surgery.
They stated that the method is a safe and effective therapeutic strategy for patients with impaired physiologic reserve and/or highly contaminated wounds. Also, its stabilizing characteristics allow for post-debridement extubation, reducing the need for prolonged paralysis and mechanical ventilation.
Disadvantages encountered using the VAC system are relatively few. Some patients reported pain when sub atmospheric pressure was applied to their sternal wounds. In our experience this was easily relieved by applying the pressure slowly.
However, overall the VAC foam dressing is considered as more comfortable than conventional gauze dressings. Excessive ingrowth of granulation tissue into the foam dressing can occur when not changed after 3 days. Thereafter, removal of dressing disrupts the newly formed granulation tissues. This can be avoided by ensuring that the patient has regular changes of dressings every 2–3 days.
Several dangerous events happened with our previous conventional policy that made us think of the VAC system, like profuse bleeding following injuries to the left internal mammary artery (LIMA) grafts or rupture of the right ventricle occurred by a shearing force between the debrided sternum and the heart. Since the use of the VAC system, the sealed vacuum prevents desiccation of the LIMA graft and the myocardium and the placement of the sponge between the right ventricle and the overlying sternal elements prevents these sternal edges from adhering to the anterior wall of the right ventricle and pulling it apart.Moreover, the vacuum sponge apparatus acts as a mechanical sternal stabilizer as well.
This allows for post-debridement extubation and reduces the need for prolonged paralysis and mechanical ventilation. Even when coughing, the patient’s chest and mediastinum have a stable relationship, this stabilization also facilitates early post-operative ambulation with the VAC in place.
We conclude from our limited experience in the treatment of 16 patients with poststernotomy DSWI by VAC that this modality of treatment proved to be an excellent alternative to conventional open and closed management of these wounds. Major plastic surgical procedures like myocutaneous muscle flap treatment can be avoided. The treatment and hospitalization times, and morbidity are reduced with appropriate compliance and equipment, thereby reducing hospitalization costs and improving quality of life for patients.
Limitations of the study
Our study was a retrospective one, with the two groups operated upon in series by different surgeons. The sample size was small and therefore, significant differences in outcome have to be interpreted with care. It would be necessary to balance the different patients and wound variables. Since both series do not cover the same time frame, the conclusions remain hazardous. Undoubtedly, a prospective, randomized trial would provide more definitive conclusions regarding superiority of a particular technique.
References
1.
Taylor GJ, Mikell FL, Moses HM, et al. Determinants of hospital charges for coronary artery bypass surgery: The economic consequences of postoperative complication. Am J Cardiol 1990; 65: 309-313.
2.
Mossad SB, Serkey JM, Longworth DL, et al. Coagulase-negative staphylococcal sternal infections after open heart operations. Ann thorac Surg 1997; 63: 395-401.
3.
El Oakley RM, Wright JE. Postoperative mediastinitis: Classification and management .Ann Thorac Surg 1996; 61:1030-1036.
4.
Abboud CS, Way SB, Balter VT. Risk factors for mediastenitis after cardiac surgery. Ann Thorac Surg 2004; 77: 676-683.
5.
Wouters R, Wellens F, Yanermen H, et al. Sternitis and mediastinitis after coronary bypass grafting. Analysis of risk factors.Tex heart inst J 1994; 21: 183-188.
6.
Milano CA, Kesler K, Archibald N, et al. Mediastinitis after coronary artery bypass graft surgery: Risk factors and long-term survival. Circulation1995; 92: 2245-2251.
7.
Francel TJ, Kouchoukos NT. A rational approach to wound difficulties after sternotomy: Reconstruction and long-term results. Ann Thorac Surg 2001; 72: 1419-1429.
8.
Borger MA, Rao V, Weisel RD, et al. Deep sternal wound infection: Risk factors and outcomes. Ann Thorac Surg 1998; 65: 1050-1056.
9.
Milano CA, Kesler K, Archibald N, et al. Mediastinitis after coronary artery bypass graft surgery. Risk factors and long term survival. Circulation 1995; 92: 2245-2251.
10.
Fuchs U, Zitterman A ,Stuettgen B ,et al. Clinical outcome of patients with deep sternal wound infection managed by vacuum-assisted closure compared to conventional therapy with open packing:Aretrospective analysis. Ann Thorac Surg 2005; 79: 526-531.
11.R
and RP, Cochran RP, Aziz S. Prospective trial of catheter irrigation and muscle flaps for sternal wound infection. Ann Thorac Surg 1998; 65: 1046-1049.
12.
Ringelman PR, Vander KC, Cameron D, et al. Long term results of flap reconstruction in median sternotomy wound infections. Plast Reconstr Surg 1994; 93: 1208-1214.
13
.Argenta LC, Morykwas MJ. Vacuum-assisted closure: A new method for wound control and treatment: clinical experience. Ann Plastic Surg 1997; 38: 563-576.
14.
Cowan KN, Teague L, Sue SC, et al. Vacuum-assisted wound closure of deep sternal infections in high-risk patients after cardiac surgery. Ann Thorac Surg 2005; 80(6): 2205-2212.
15.
Mullner T, Mr Konjic L, Kwasny O, Vecsei V. The use of negative pressure to promote the healing of tissue defects: A clinical trial using the vacuum sealing technique. Br J Plast Surg 1997; 50(3): 194-199.
16.
Immer FF, Durrer M, Mühlemann KS, et al. Deep sternal wound infection after cardiac surgery: Modality of treatment and outcome. Ann Thorac Surg 2005; 80(3): 957-961.
17.
Sjögren J, Gustafsson R, Nilsson J, et al. Clinical outcome after poststernotomy mediastinitis: Vacuum-assisted closure versus conventional treatment. Ann Thorac Surg 2005; 79(6): 2049-2055.
18.
Gustafsson RI, Sjögren J, Ingemansson R. Deep sternal wound infection: a sternal-sparing technique with vacuum-assisted closure therapy .Ann Thorac Surg 2003; 76(6): 2048-2053.
19.
Fleck TM, Fleck M, Moidl R, et al. The vacuum-assisted closure system for the treatment of deep sternal wound infections after cardiac surgery. Ann Thorac Surg 2002; 74(5): 1596-1600.
20. The Clinical Advantage, www.woundvac.com.