Objective: To evaluate the potential value of 16-slice multidetector computed tomography to non-invasively detect significant coronary in-stent restenosis.
Methods: During a period of 9 months (between 1/6/2005 to 22/3/2006), fifty patients (37 (74%) male, 13 (26%) female, mean age 57.8 ± 11.3 years) previously subjected to percutaneous implantation of coronary stents before mean of 7.8 ± 2.3 months, underwent a 16-row multidetector computed tomography for suspected in-stent restenosis. The mean time between multidetector computed tomography and repeat invasive coronary angiography was 3.6 weeks. The scan was completed in <20 seconds. Multiplanar reconstructions were made for all stented coronary artery segments. Stents were viewed in long and short axes and classified according to graded visual analysis of luminal contrast attenuation into either being patent (lumen reduction <50%) or having in-stent restenosis (lumen reduction ≥ 50%). The diagnostic performance of multidetector computed tomography was evaluated with invasive coronary angiography serving as the standard of reference. Results from the two angiographic techniques were compared after performing four separate analyses: First an in-stent analysis, second an in-segment analysis, third a patient-based analysis and lastly an in-stent analysis but after excluding stents with total in-stent occlusion (ISO). The effects of stent length, diameter and strut thickness were also analyzed.
Results: A total of 80 stented coronary vessel segments were screened for in-stent restenosis using multidetector computed tomography and then compared with invasive coronary angiography. In-stent restenosis (≥ 50% luminal narrowing by quantitative coronary angiography) was found on invasive coronary angiography in twenty one (26%) stented segments (13 restenoses and 8 total occlusions) in 18 (36%) patients. The sensitivity, specificity, positive predictive value and negative predictive value of the multidetector computed tomography for the detection of coronary ISR were 71.4%, 88.1%, 68.2% and 89.7% respectively, compared to invasive coronary angiography restenosis. This allowed confirmation of stent patency in 53/59 (89.7%) stents and correct identification of in-stent restenosis and occlusion in 15/21(71.4%) stents. False negative results occurred in 6 (7.5%) stents and false positive results in another 6 (7.5%) stents.
Conclusion: In-stent restenosis can be diagnosed with moderate sensitivity using 16-row multidetector computed tomography. The high negative predictive value implies a significant role in excluding in-stent restenosis. Further improvements in spatial and temporal resolution of multidetector computed tomography are still required to challenge invasive angiography and become suitable for clinical implementation.
Key words: In-stent restenosis, computed tomography, 16- multidetector computed tomography.
JRMS Dec 2007; 14(3): 5-11 IntroductionCoronary stenting currently represents the default strategy during percutaneous coronary interventions since it has been shown to have superior short and long-term outcomes compared to conventional balloon angioplasty.(1,2,3)
Angiographic and clinical restnosis after stenting develops in 15-45% of the cases and constitutes a major limitation to the effectiveness of the technique.(1,2) Despite the introduction of drug eluting stents, in-stent restenosis (ISR) remains a major issue in follow up.(4)
Several reports(5,6) have shown that approximately 50% of patients remain asymptomatic when ISR occurs; thus, chest pain after coronary stenting is a poor indication of ISR. Therefore a simple and noninvasive method for late ISR might help to select patients who require further angiographic evaluation.
Non-invasive detection of ISR is a real problem: exercise stress tests,(7) stress echocardiography, and myocardial scintigraphy(5,8) have poor sensitivity and specificity in this aspect.
The impairment of endothelium-dependent flow-mediated dilation (FMD) of the brachial artery(7,9) has been recently found to be independently associated with late ISR in native coronary arteries .The combined assessment of chest pain, positive exercise test and FMD have an incremental effect on the sensitivity and specificity in this regard.(7)
Cardiac imaging with multidetector computed tomography (MDCT) is rapidly evolving to become an alternative to catheter-based invasive coronary angiography (ICA), which is not without risk and complications. While visualizing stent lumen proved to be non- practical by 4 slice MDCT(10,11) and even 16 –slice MDCT,(12) only a limited number of studies reported the feasibility of analyzing stents by 16 slice MDCT.(12-14)
This report presents our experience with MDCT in the diagnosis of ISR in a patient cohort with history of implanted stents who were followed up at Queen Alia Heart Institute (QAHI) and scheduled for repeat invasive coronary angiography (ICA) due to recurrent angina and clinical suspicion of ISR.
MethodsA 16-row MDCT has become available at KHMC in mid 2004. Because these scanners have the potential to allow noninvasive coronary angiography, we aimed to investigate this potential value in a group of patients with previously implanted coronary stents who underwent follow-up coronary angiography for suspected ISR.
Our study comprised fifty patients (37(74%) males, 13 (26%) females, mean age 57.8 ± 11.3 years) collected during a period of 9 months (between 1/6/2005 to 22/3/2006), who were subjected to 16-row MDCT to rule out ISR after mean of 7.8 ± 2.3 months from stent implantation. Invasive coronary angiograms were done later on according to the standard techniques. The mean elapsing time between MDCT and ICA (MDCT-CA) was 3.6 weeks. Patient characteristics are summarized in Table I.
Table I: Patient characteristics
|
|
Total
N(%)
|
Male
N(%)
|
Female
N(%)
|
|
No
of patients
|
50
|
37 (74)
|
13 (26)
|
|
Age
|
57.8 ± 11.3
|
56.3 ± 12.9
|
62.3 ± 9.2
|
|
Smoking
|
31 (63)
|
29 ( 78)
|
2 ( 15)
|
|
Diabetes
|
24 (48)
|
15 ( 41)
|
9 ( 69)
|
|
Hypertension
|
29 (58)
|
18 ( 49)
|
11 (85)
|
|
Family
history
For
CAD
|
9 ( 18)
|
7 (19)
|
2 (15)
|
|
Hyperlipidemia
|
29 (58)
|
20 ( 54)
|
9 (69)
|
Variables are expressed with their mean ± standard deviation.
Categorial data are presented with absolute numbers and Percentages
The patients were placed within the gantry of a 16-row MDCT scanner (Light SpeedTM 4.0GE Medical Systems, USA). The scan parameters were: 16 x 0.625 mm Collimation; tube rotation time 500 ms; tube voltage 100kV; tube current 420 mAs. No tube modulation was used. Patients with a heart rate above 70 beats /min received a single oral dose of 50-200 metoprolol 1-2 hours before the scan unless contraindicated (overt heart failure or bad pulmonary function).
Data were acquired during a breathhold of 20 seconds and were sent to a separate workstation (ADW 4.0, GE, USA) that was used to reconstruct the images using the standard built-in retrospective ECG-dependent reconstruction algorithms. Images were viewed in two dimensional curved multiplanar reformats (MPR) in long axis and short axis perpendicular to the centerline (see Fig. 1 & 2).
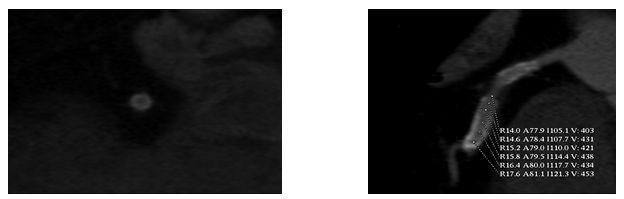 Fig. 1:
Fig. 1: Short and long axes views of stented left anterior descending artery showing dense intraluminal contrast enhancement within the stent homogenous to the density of reference vessel indicative of stent patency. Measured > 430 Housefield units by densitometric analysis
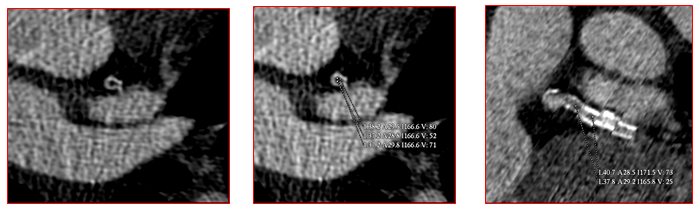 Fig. 2:
Fig. 2: Markedly decreased intraluminal density with absent contrast enhancement distal to circumflex stent indicative of total in-stent occlusion. Measured only 50-80 Housefield units by densitometric analysis
Graded visual analysis was performed to measure luminal enhancement and compare it inside and outside the stented segment at various points along the stent. ISR was considered to be absent, when the contrast density in the stent lumen was almost homogenous and similar to the density in the reference vessels visually. All scans were analyzed by one observer (NA) blind to the results of ICA and to the history of the patient.
Exclusion criteria included: (1) stented saphenous vein grafts, (2) patients with irregular rhythm, (3) those unable to perform a 20 sec breath-hold, (4) those presenting with acute coronary syndrome or (5) those that have contraindications to iodinated contrast material.
Four analyses were performed: An in-stent analysis confined to the portion of the artery covered by the stent and an in-segment analysis including the stent and 5 mm proximal or distal to the stent edges. A patient based analysis was also performed. A patient was considered to have restenosis by ICA or MDCT if restenosis was present in any stented segment for either modality. A separate fourth analysis was performed after excluding totally occluded stents since the diagnostic accuracy of MDCT may be greater for total rather than subtotal stent occlusion.
The effects of various factors including stent length, diameter, and strut thickness on the correlation between the two angiographic modalities were also analyzed.
Invasive coronary angiograms were done according to the standard techniques. Quantitative coronary measurements were performed on all cineangiograms by a single experienced observer (AO) unaware of the MDCT results with manual caliper using the outer diameter of the contrast-filled guiding catheter tip as the calibration reference standard. Paired cine frames of two orthogonal views in the end-diastolic frames showing the stenosis in its most severe projection were selected. ISR was defined if the mean lumen diameter reduction was ≥ 50%-100%. All segments ≥ 2mm were included for comparison with MDCT.
Results The diagnostic performance of MDCT was evaluated with ICA serving as the standard of reference. Results from the two angiographic techniques were compared. Results were presented as sensitivity, specificity, positive predictive value and negative predictive value (Table II).
Table II:Detection of significant in-stent stenosis with 16-Row multidetector computed tomography coronary angiography.
|
|
|
MDCT
|
|
|
|
|
|
|
|
ISR detection
|
CCA
Total (%)
No/Yes
|
TN
|
TP
|
FN
|
FP
|
Sensitivity
|
Specificity
|
PPV
|
NPV
|
P
value
|
Kappa value
|
|
In-stent
analysis
|
80(100)
59/21
|
53
|
15
|
6
|
6
|
71.4%
|
89.8%
|
68.2%
|
89.7%
|
0.000
|
0.62
|
|
Stent
length
≥ 20mm.
|
28 (35)
18/10
|
17
|
6
|
4
|
1
|
60%
|
94.4%
|
85.7%
|
81%
|
0.02
|
0.58
|
|
Stent
Length
<
20mm
|
52 (65)
41/11
|
36
|
9
|
2
|
5
|
81.8%
|
87.7%
|
64.3%
|
94.7%
|
0.05
|
0.32
|
|
Stent
diameter
<
3mm
|
30 (38)
20/10
|
20
|
8
|
2
|
0
|
80%
|
100%
|
100%
|
91%
|
0.000
|
0.83
|
|
Stent diameter
≥3mm
|
50 (62)
39/11
|
33
|
7
|
4
|
6
|
63.6%
|
84.6%
|
53.8%
|
89.2%
|
0.01
|
0.45
|
|
Stent
Type
Express
2
|
34 (43)
22/12
|
20
|
7
|
5
|
2
|
58.3%
|
90.9%
|
77.8%
|
80%
|
0.01
|
0.51
|
|
Stent
Type Penta/Zeta
|
46 (57)
37/9
|
33
|
8
|
1
|
4
|
88.9%
|
89.2%
|
66.7%
|
97.1%
|
0.001
|
0.69
|
|
Stent
diameter
<2.75mm
|
12 (15)
9/3
|
9
|
3
|
0
|
0
|
100%
|
100%
|
100%
|
100%
|
0.000
|
1.0
|
|
Stent
diameter
≥2.75mm
|
68 (85)
50/18
|
44
|
12
|
6
|
6
|
66.7%
|
88%
|
66.7%
|
88%
|
0.001
|
0.54
|
FN: false negative, FP: false positive, PPV: positive predictive value, TN: true negative, NPV: negative predictive value, TP: true positive.
Table III: Stent analysis
|
|
Total N(%)
|
Male
N(%)
|
Female
N(%)
|
|
Number
of Stents
|
80
|
61(76)
|
19(24)
|
|
Stented
segments
LAD
DL
Cx
Branch Cx
RCA
|
31 (39)
2 (2)
12 (15)
11 (14)
24 (30)
|
23 ( 38)
1 ( 2)
10 (16)
10 (16)
17 (28)
|
8 (42)
1 (5)
2 (11)
1 (5)
7 (37)
|
|
Stent
type ”Express”
|
34 (43)
|
24 (30)
|
10 (53)
|
|
Stent
length
≥ 20mm.
|
28 (35)
|
22 (36)
|
6 (32)
|
|
Stent
diameter
<
3mm
≥3mm
|
30 (38)
50 (62)
|
20 (33)
41 (67)
|
10 (53)
9 (47)
|
|
Stent
diameter
<2.75mm
≥2.75mm
|
12 (15)
68 (85)
|
8 (13)
53 ( 87)
|
4 (21)
15 (79)
|
LAD: left anterior descending, DL: diagonal, Cx: circumflex, RCA: right coronary artery.
Statististical analysis was done using one way Anova. Measures of association were calculated using Pearson Chie square. A р-value of less than 0.05 was regarded as significant. Measures of agreement were calculated using Kappa values.
Patient Characteristics
Demographic data are given in Table I these are noteworthy for male preponderance (74%) and the prevalence of coronary risk factors (> 50% are smokers, hypertensives and diabetics). Mean age was 57.8 ± 11.3 years (56.3 ± 12.9 years for males and 62.3 ± 9.2 years for females).
Stent Analysis
Stent type and parameters are given in Table III, including stent length and diameter. Stents used were only bare metal stents (BMS) that were almost equally divided between the major coronary vessels (left anterior descending artery, right coronary artery and the left circumflex artery). 35% were long stents (> 20mm in length), 62% were ≥ 3mm, 85% were ≥ 2.75mm and only 15% were < 2.75mm in diameter.
Table IV:In-segment, per-patient analyses and analysis excluding total in-stent occlusion
|
|
|
MDCT
|
|
|
|
|
|
|
|
ISR
detection
|
CCA
Total (%)
No/Yes
|
TN
|
TP
|
FN
|
FP
|
Sensitivity
|
Specificity
|
PPV
|
NPV
|
P
value
|
Kappa value
|
|
In-segment
analysis
|
80 (100)
|
52
|
14
|
7
|
7
|
66.7%
|
88.1%
|
66.7%
|
88.1%
|
0.000
|
0.55
|
|
Per
patient analysis
|
50(100)
|
25
|
14
|
4
|
7
|
77.8%
|
78.1%
|
66.7%
|
86.2%
|
0.000
|
0.54
|
|
All
stents excluding
ISO
|
72
59/13
|
53
|
8
|
5
|
6
|
61.5%
|
89.8%
|
71.4%
|
89.8%
|
0.001
|
0.5
|
Used stents were only stainless steel bare metal stents that were almost equally divided between two Guidant manufactured stents (Penta and Zeta) and Boston scinentific manufactured stents (Express 2) stents. Both groups of stents have strut thickness of 0.0049 inch and 0.0052 inch respectively.
Angiographic FindingsUsing ≥ 50% cutoff values for clinically significant instent restenosis, ISR was diagnosed By QCA in 21 stents (26.3%), in 18 patients (36%). Total in-stent occlusion was found in 8 stents (10%).
Comparison between MDCT and ICA
When in-stent analysis was performed, the presence of ISR was correctly identified in 15 of 21 restenotic lesions, (sensitivity 71.4%). The absence of stenosis was correctly identified in 53 of 59 segments (specificity 89.8%). The positive predictive value was 68.2% and the negative predictive value was 89.7%.
Therefore, the correlation of MDCT compared with that of ICA in our cohort of patients was modest (Kappa=0.62), except for right coronary artery where MDCT performed badly both in terms of statistical significance and kappa value (p value=0.1, Kappa=0.40).
Our data showed that MDCT performed better in diagnosing ISR when stents are longer than 20mm (P value 0.02, kappa 0.58) than when they are shorter (p value 0.05, kappa = 0.32). Surprisingly, however MDCT of small diameter stents in our series had better agreement with ICA. All stents that were < 2.75 mm in diameter (n==12) were diagnosed correctly by MDCT as being restenotic or free from restenosis with no false positive or negative results (kappa value of 1.0).
Our results were comparable to the results of many previous trials(14,16) where better correlation was found between MDCT and ICA with stents that had reportedly lower strut thickness. The presence of ISR was more easily recognizable on MDCT with Guidant stents versus Express 2 stents (Sensitivity 88.9% versus 58.3%). NPV fell by 17.1% and the agreement fell by 0.18 with Express 2 stents (Kappa =0.51 vs. 0.69 for Penta and Zeta stents). Although strut material is known to play a more important role in this regard, comparison between our both stent models was not feasible as both are made of the same material (stainless steel).
Marginally better sensitivity; specificity; PPV and NPV were obtained when analysis was restricted to in-stent rather than in-segment (see Table IV). Better detection of in-stent vs. in-segment restenosis may be related to beam hardening artifacts sometimes present immediately adjacent to stent edges leading to under-or overdiagnosis of defects at these points. In our study, one additional missed and one additional over-diagnosed instent restenosis was obtained with in-segment analysis by MDCT.
On a patient-related basis, better sensitivity (77.8%) but poorer specificity (78.1%) was obtained with per patient analysis vs. in-stent analysis (see Table IV).
Diagnostic accuracy of MDCT may be greater for total rather than subtotal occlusion. Both sensitivity and agreement of MDCT with ICA results went down after excluding totally occluded stents from our analysis (Sensitivity 61% vs. 71%, kappa= 0.5 vs. 0.62).
Discussion Coronary stents have been notoriously difficult to assess by computed tomography. Multiple difficulties contribute to the inability to visualize the stent lumen:(15) (1) the small diameter of coronary arteries, (2) partial volume effects and beam hardening and (3) cardiac motion artifacts (banding artifacts). The blooming effect is a fairly constant phenomenon, which is related to high-density artifacts created by the stent struts. This effect tends to decrease with large diameter stents. Assessment is further complicated by lower contrast-to-noise, and vessel wall calcifications.
With the advent of submillimeter 16-slice MDCT scanners (slice thickness 0.625 mm), more sophisticated reconstruction algorithms decreased the beam hardening of stents greatly. Moreover, the motion artifacts due to respiration and heartbeats were also decreased by the shorter scan time (<20 seconds).
Despite the rapidly evolving improvements in MDCT spatial resolution, severe artifacts found with tantalum (e.g.: Wiktor) or gold-coated stents (e.g., Bestent2) or with covered stent grafts render stent lumen analysis by MDCT un-interpretable.(13,15)
Cardiac motion artifacts are more likely to limit proper assessment of the stents implanted in the right coronary arteries. In our series, correlation between MDCT and ICA regarding ISR was optimal in the circumflex and diagonal artery (Kappa= 1.0) and was worst in right coronary artery (Kappa= 0.4), which is mostly explained by the influence of motion artifacts.
Stents with thicker struts are more prone to high-density artifacts and poor assessability.(14) Schuijf et al(14) considered strut thickness < 140 µm as a threshold at which struts were deemed thick and more prone to high density artifacts. Compared to previously reported trials, it’s clear that in our series MDCT/ICA correlation was much better with stents that have lower strut thickness (Penta and Zeta: 124.5 µm) than those with higher one (Express2: 132.1 µm), although both stent types were below the mentioned threshold.
The determining factor for lumen interpretation is stent size.(16) However, our results are inconsistent with most previous trial results where MDCT performs better when the stent diameter is more than 3mm; the diameter at which asssessability by MDCT is not affected by the blooming effects, regardless of the strut thickness.(16) Our results showed that all stents with <2.75 mm in diameter (n=12) were diagnosed correctly by MDCT as being restenotic or free from restenosis with no false positive or negative results (kappa value of 1.0). However no conclusion can be made out of that as their number is relatively small (n=12/80; 15%).
The percentage of patients with angiographic ISR in our series is relatively high (36%). The design of study, which was not a follow up study but included all patients referred for repeat coronary angiography on ischemia-driven basis, may account for this high restenosis rate. Therefore our data concerning the sensitivity for detecting ISR could be interpreted with good amount of confidence.
When in-stent analysis was performed in this study, the presence of ISR was correctly identified in 15 of 21 restenotic lesions. (Sensitivity 71.4%). This moderate sensitivity was associated with relatively low PPV (68.2% stent-based, 66.7% patient-based). Our obtained specificity and NPV though were high whether with in-stent in-segment or with per patient analyses, making the current MDCT a potentially useful screening test for exclusion of ISR.
Totally occluded stents are more easily recognizable on MDCT.(11) The higher the prevalence of total occlusions in the population examined, the greater the sensitivity and PPV of MDCT is likely to be. Eight stented segments were totally occluded in the present study comprising 38% of the total re-stenotic lesions. MDCT diagnosed 7 of these correctly. Among the remaining 13-restenotic lesions that were not totally occluded, correct diagnosis by MDCT was given in only 8 cases. A repeat analysis after exclusion of totally occluded segments showed worsened the yield of MDCT in this application, see Table IV.
On a patient-related basis, MDCT excluded ISR in 25 out of 32 patients, but missed 4 out of 18 patients (22.2%) with angiographic ISR. Therefore, for a patient population similar to that of the present study with highly prevalent angiographic ISR (36%), if the decision to perform ICA were to rely solely on MDCT, this would result in only 2 in 10 patients with restenosis being missed.
This study showed that in-stent restenosis could be diagnosed with moderate sensitivity using the 16-slice MDCT scanner. The high NPV implies a significant role for MDCT in excluding ISR. Applicability of MDCT to an asymptomatic stent population or in a population with lower prevalence of ISR (era of drug-eluting stents) may yield different results. Further research in these areas is needed to explore these diversities.
References
1.
Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med 1994; 331:496-501.
2.
Versaci F, Gaspardone A, Tomai F, et al. A comparison of coronary-artery stenting with angioplasty for isolated stenosis of the proximal left anterior descending coronary artery. N Engl J Med 1997; 336:817-822.
3.
Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon expandable stent implantation with balloon angioplasty in patients with coronary artery disease: the BENESTENT study group. N Engl J Med 1994; 331:489-495.
4.
Sousa JE, Costa MA, Sousa A, et al. Two-year angiographic and intravascular ultrasound follow-up after implantation of sirolimus-eluting stents in human coronary arteries. Circulation 2003; 107:381-391.
5.
Giedd KN, Bergmann SR. Myocardial perfusion imaging following percutaneous coronary intervention: the importance of retenosis,disease progression,and directed reintervention. J Am Coll Cardiol 2004; 43:328-336.
6.
Ruygrok PN, Webster MW, de Valk, et al. Clinical and angiographic factors associated with asymptomatic restenosis after percutaneous coronary intervention .Circulation 2001; 104:2289-2294.
7.
Kitta Y, Nakamura T, Kodama Y, et al. Endothelial vasomotor dysfunctio in the brachial artery is associated with late in-stent coronary restenosis. J Am Coll Cardiol 2005; 46: 648-655.
8.
Zellweger MJ, Weinbacher M, Zutter AW, et al. Long-term outcome of patients with silent versus symptomatic ischemia six months after percutaneous coronary intervention and stenting. J Am Coll Cardiol 2003; 42:33–40.
9.
Wu TC, Chen YH, Chen JW, et al. Impaired forearm reactive hyperemia is related to late restenosis after coronary stenting. Am J Cardiol 2000; 85: 1071-1076.
10.
Maintz D, Grude M, Fallenberg EM, et al. Assessment of coronary arterial stents by multislice-CT angiography. Acta Radiol 2003; 44: 597-603.
11.
Kruger S, Mahnken AH, Sinha AM, et al. Multislice spiral computed tomography for the detection of coronary stent restenosis and patency. International Journal of Cardiology 2003; 89:167-172.
12.
Cademartiri F, Marano R, Runza G, et al. Non-invasive assessment of coronary artery stent patency with multislice CT: preliminary experience. Radiol Med 2005; 109: 500-507.
13.
Nieaman K, Cademartiri F, Raaijmakers R, et al. Noninvasive angiography evaluation of coronary stents with multi-slice spiral computed tomography. Herz 2003; 28:136–142.
14.
Schuijf JD, Bax JJ, Jukema W, et al. Feasibility of assessment of coronary stent patency using 16-slice computed tomography. Am J Cardiol 2004; 94:427–430.
15.
Kitagawa T, Fujii T, Yasuyuki Tomohiro Y, et al. Noninvasive assessment of coronary stents in patients by 16-slice computed tomography. International Journal of Cardiology 2006; 109: 188-194.
16.
Gilard M, JC Cornily JC, Pennec PY, et al. Assessment of coronary artery stents by 16 slice computed tomography. Heart 2006; 92: 58-61.