Abstract
Objectives: Transcatheter
closure of patent ductus arteriosus is a well-established procedure. The aim of
this study was to assess the medium term results of patent ductus arteriosus
closure using the Amplatzer Duct Occluder.
Methods: From January
1998 to January 2005, 204 cases (77 males and 127 females) underwent an attempt
of transcatheter closure of their patent ductus arteriosus at Queen Alia Heart
Institute using the Amplatzer Duct Occluder TM device. Their median
age was 3.5 years (range 0.8-13 years), their median weight 14kg (range
6-32kg), their mean Qp/Qs was 2.3±0.6, their mean systolic pulmonary artery
pressure was 38.44±7mmHg. The mean narrowest diameter of the pulmonary end of
the patent ductus arteriosus angiographically was 4.2±0.8mm (range 3-8mm). The
devices used were (6-4, 8-6, 10-8 and 12-10mm) delivered antegrade via 5-7
French sheaths. All patients had chest X-ray and color flow echocardiographic
follow-up at 24 hours, one, three, six months and yearly thereafter.
Results: There was
immediate and complete closure of the ductus in 180 (88.24%) of cases. The
remaining 24 (11.76%) patients had a trivial residual shunt through the device
mesh. Follow-up color flow Doppler echocardiography revealed complete closure
of patent ductus arteriosus in 96% of cases at 24 hours, and complete closure
at one month follow-up in 100% of cases. One patient developed aortic
obstruction where the duct joined the aorta at a more acute angle, which was
retrieved surgically. Otherwise no other complications were reported. Neither
thromboembolization nor hemolysis or recanalization of the ductus was reported.
Furthermore, chest radiographs and Doppler echocardiography follow up revealed
no evidence of wire fracture or device disruption or any episodes of infective
endocarditis.
Conclusion: Since the initial clinical experience in 1998, the
transcatheter closure of patent ductus arteriosus using the Amplatzer Duct
Occluder has proven to be an easy procedure that could be mastered quickly but
with caution and at the same time it is an effective procedure that has almost replaced
surgery in our center. Longer follow up
will be needed to precisely define the safety and indications of this device.
Key words: Amplatzer Duct
Occluder, Patent ductus arteriosus, Medium term follow up
JRMS
August 2008; 15(2): 15-18
Introduction
Isolated patent ductus
arteriosus (PDA) is the second most common congenital heart disease(1)
accounting for 10-18% of cardiovascular malformations.(2)
Closure of PDA even with small shunt volumes in asymptomatic patients is
recommended because of the risk of endocarditis (1.5% per year) and the
potential development of congestive heart failure or pulmonary hypertension.(3)
Transcatheter occlusion of persistent ductus arteriosus has replaced surgery as
the treatment of choice(4) and became a well established
technique with low morbidity and no reported mortality.(1,5)
The aim of the study was to assess the short and intermediate results of the
transcatheter closure of PDA using the self expandable, self centering and
repositionable Amplatzer Duct Occluder Device (ADO) at Queen Alia Heart
Institute (QAHI).
Methods
From January 1998 to
December 2005, 204 patients (77 males and 127 females) with PDA underwent an
attempt of transcatheter closure of their PDA at QAHI using the ADO device. Their median
age was 3.5 years (range 0.8-13 years), their median weight 14 Kg (range
6-32Kg). All patients had clinical and echocardiographic evidence of a PDA.
Patients who were excluded from the study are those who had small PDA’s <3mm
who underwent coil closure and those with large PDA’s >8mm who were sent for
surgery. Most of our patients were asymptomatic, 15 patients presented with
failure to thrive, and 45 patients with recurrent respiratory symptoms.
Informed consent was obtained from all patients or their guardian.
The ADO device that was used is a self
expandable, mushroom-shaped device that has been described in details in
previous studies(5,6) (Fig. 1).
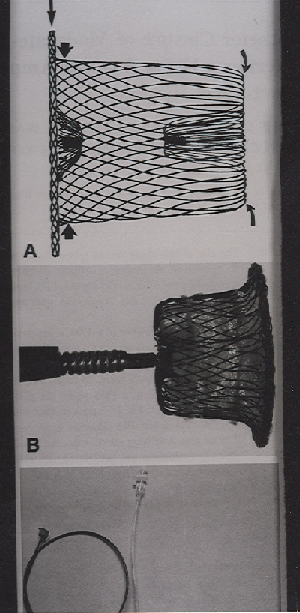
Fig. 1. The Amplatzer Duct Occluder device
|
The sizes of the devices
that were used were: 6-4, 8-6, 10-8 and 12-10mm, which were delivered
antegradely via 5-7 French sheath. The technique of transcatheter closure was
similar to that described by Masura et al.(6,7) (Fig.
2,3,4).
 |
|
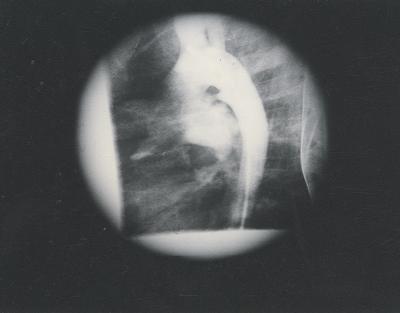
Fig. 2. Angiogram in the descending aorta shows the patent ductus arteriosus between the descending aorta and the left pulmonary artery 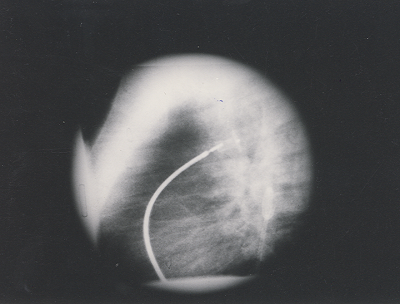
Fig. 3. Angiogram in the descending aorta shows the deployment of both aortic and pulmonic discs of the device in the ampulla of the ductus 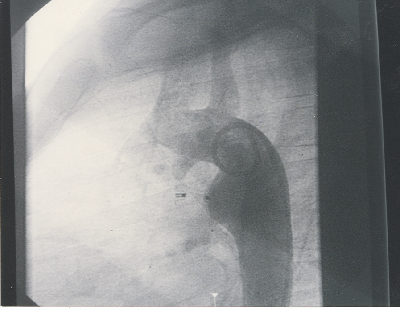
Fig. 4. Angiogram in the descending aorta after release of the Amplatzer Duct Occluder device shows complete closure of the ductus arteriosus |
Prophylactic antibiotics
with second-generation cephalosporin were given during the procedure to every
patient in a dose of 25mg/kg every eight hours for three doses. After 24 hours
the patient was discharged home on no medications. Follow up of patients was
done by doing serial Chest X-Rays in postero-anterior and lateral positions,
and 2D-echo with color flow mapping which were done at 24 hours, one, three and
six months after the closure then yearly thereafter in order to assess;
position of the device, presence of residual ductal flow, recanalization, left
pulmonary artery stenosis, coarctation of the aorta, and wire fractures.
Results
According to the classification that was
adopted by Krichenko et al.(8) One hundred sixty seven
patients had PDA type A, two patients had PDA type B, 17 patients had PDA type
C, six patients had PDA type D and 12 patients had PDA type E. The mean
systolic pulmonary artery pressure was 38.44±7mmHg. The mean Qp/Qs was
2.3±0.6.The mean narrowest diameter of the pulmonic end of the PDA
angiographically was 4.2±0.8mm. Complete and immediate angiographic closure was
present in 180 patients (88.24%).The remaining 24 patients had a trivial
residual shunt. In one patient, who had a PDA type E according to Krichenko et
al. classification, the duct joined the aorta at more acute angle than usual,
so during the deployment of the aortic retention disc it ended projecting into
the aortic lumen, obstructing more than half of its lumen, which was confirmed
by 2D-echo and was retrieved later on surgically. This patient was excluded
from our follow up plan. No patient required blood transfusion. At 24 hr, color
Doppler flow revealed complete closure in 196 patients (96%). At one month
follow up it showed a complete closure in the 203 patients (100%). Follow up data were available to 195 patients
at three, six months after the closure, and yearly thereafter with no evidence
of wire fracture, device migration or recanalization, evidence of hemolysis, thromboembolism
or endocarditis. The symptomatic patients improved dramatically after the
closure; regarding their weight gain and respiratory symptoms.
Discussion
Since Porstmann et al.
success in closing PDA by transcatheter route using an Ivalon plug in 1969,(6,9)
numerous different occluding devices and coils have been used with satisfactory
results,(10,16) leaving the conventional surgical ligation of
the PDA reserved for symptomatic preterm infants or large ducts.(17) The ideal device has been chased for decades
since Porstmann et al. introduced the Ivalon plug nearly 37 years ago.
The ideal device should possess the ‘wish-list’ characteristics(18)
including: low cost, ease of delivery using smaller introducer sheaths,
should achieve high complete closure rate, lower morbidity and mortality
compared to surgical closure.(16-18) Rashkind occluder device was once accepted
and used widely, but its high cost, the large transvenous sheath, the high
incidence of late residual shunt with the significant risk of LPA stenosis led
to the continuous search for alternatives.(10,12,17,18) Recently, Gianturco coils have been used on a
wide scale to close PDA’s non-surgically due to its low cost, ease of delivery
using smaller catheters, and its high closure rate approaching 98%-100%.(5,16,18) The coil has disadvantages, the most important one is the lack of a
controlled-release mechanism, that once they are released they cannot be
retrieved back. Recently,
controlled-release coils have been developed(19) and are
available for use, but still not in our center. Another disadvantage is its
unsuitability for use in large PDAs. So,
the drawbacks of these devices namely; the high incidence of residual shunts,
the sometimes complex delivery system and their unsuitability for large PDA’s
and infants(6,18,20) had led to the newly introduced ADO device
which has a number of plausible features;(17) namely retrievability up to the point of
deployment and the ease of the delivery system using 5F to 7F long sheath, its
suitability for all sized PDA’s.(5,17,21) Several studies reported the immediate and
short term results of transcatheter closure with ADO regarding the 100%
occlusion rate, yet still significant complications had occurred including
death, hemolysis, device embolization, device misplacement and significant
blood loss during the procedure.(10,17,18,22) In our series
of 204 patients the closure was successful in all of our patients. One
significant complication had occurred as mentioned before; the device had
obstructed more than half of the aortic lumen causing pseudo coarctation,
constituting 0.97% of patients which could have been avoided with careful
selection of patients and careful implantation of the device. Otherwise no late minor or major
complications such as bleeding, hemolysis, deaths, device embolization or
displacement, and wire fracture had occurred including. The symptomatic
patients improved dramatically. The youngest patient who underwent PDA closure
was eight months of age with body weight of 6kg. Our future challenge is to try
to close PDAs in younger patients with weight of less than 5kg since the
experience in neonates and younger patients is limited worldwide.(7,17)
Conclusion
Since the initial
clinical experience in 1998, the transcatheter closure of PDA using the ADO has proven to be an
easy procedure that could be mastered quickly but with caution and at the same
time effective procedure that it has almost replaced surgery in our center. Longer follow up will be needed to precisely
define the safety and indications of this device, especially in younger
patients.
Reference
1.
Zanchetta
M, Dimopoulos K, Rigatelli G, et al. Patent ductus arteriosus closure using the new
Amplatzer Duct Occluder. Preliminary results and review of the literature. Minerva
Cardioangiol 2001; 49(6): 369-376.
2.
Chessa
M, Mohamed B, Giusti S, et al. Transcatheter treatment of patent ductus arteriosus. Ital Heart J Suppl
2002; 3 (11): 1092-1097.
3.
Chatterjee
T, Windecker S, Pfammatter JP, et al. Non-surgical closure of patent ductus arteriosus:
acute and long-term results. Schweiz Med Wochenschr 2000; 130(18): 664-670.
4.
Dalen
ML, Bjornstad PG. Transcatheter
closure of persistent ductus arteriosus. Tidsskr Nor Aegeforen 2003;
123(23): 3358-3360.
5.
Thanopoulos
B, Fakhri H, Aktham H, et al. Further experience with transcatheter closure of the patent ductus
arteriosus using the Amplatzer duct occluder. J Am Coll Cardiol 2000; 35(4):1016-1021.
6.
Masura
J, Kevin P, Thanopoulos B, et al. Catheter closure of moderate- to large-sized patent
ductus arteriosus using the new Amplatzer duct occluder: immediate and
short-term results. J Am Coll Cardiol 1998; 31: 878-882.
7.
Fischer
G, Stieh J, Uebing A, et al. Transcatheter closure of persistent ductus arteriosus in infants using
the Amplatzer duct occluder. Heart 2001; 86: 444-447.
8.
Krichenko
A, Benson LN, Burrows P, et al. Angiographic classification of the isolated,
persistently patent ductus arteriosus and implications for percutaneous
catheter occlusion. Am J Cardiol 1989; 67: 877-880.
9.
Porstmann
W, Wierny LN,
Warneke H. Closure of the
persistent ductus arteriosus without thoracotomy. Ger Med Mon 1967; 12:
259-261.
10. Butera G, De Rosa G, Chessa M, et
al. Transcatheter closure
of persistent ductus arteriosus with Amplatzer duct occluder in very young
symptomatic children. Heart 2004; 90: 1467-1470.
11. Rashkind WJ, Mullins CE, Hellenbran D,
et al. Nonsurgical closure
of patent ductus arteriosus: clinical application of the Rashkind PDA occluder.
Circulation 1987; 75: 583-592.
12. Rao PS, Sideris EB, Haddad J, et al. Transcatheter occlusion of patent ductus arteriosus
with adjustable buttoned device: initial clinical experience. Circulation
1993; 88:1119-1126.
13. Verin VE, Saveliev VS, Kolody SM, et
al. Results of
transcatheter closure of patent ductus arteriosus with the Botallo occluder. J
Am Coll Cardiol 1994; 23: 759-765.
14. Moore JW, Gearge L, Kirkpatrick SE, et
al. Percutaneous closure of
the small ductus arteriosus using occluding spring coils. J Am Coll Cardiol
1994; 23: 759-765.
15. Hijazi ZM, Gegge RL. Results of anterograde
transcatheter closure of patent ductus arteriosus using single or
multiple Gianturco coils. Am J Cardiol 1994; 74: 925-929.
16. Celiker A, Qureshi SA, Bilgic A, et
al. Transcatheter closure
of patent arterial duct using controlled- release coils. Eur Heart J
1997; 18: 450-454.
17. Bilkis A, Alwi M, Hasri S, et al. The Amplatzer duct occluder: experience in 209
patients. J Am Coll Cardiol 2001; 37(1): 258-261.
18. Faella HJ, Hijazi ZM. Closure of the patent ductus arteriosus with the
Amplatzer PDA device: Immediate results of the international clinical trial.
Cathet Cardiovasc Intervent 2000; 51: 50-54.
19. Akagi T. catheter intervention for congenital heart diseases. Kyobu
Geka 2006; 59:681-7.
20. Duke C, Chan KC. Aortic obstruction caused by device occlusion of
patent arterial duct. Heart 1999; 82: 109-111.
21. Pass RH. Amplatzer Duct Occluder device: A new technology for
the closure of the moderate-to-large-sized patent ductus arteriosus. Expert
Rev Med Devices 2006; 3(3): 291-296.
22. Masura J, Tittel P, Gavora P, Podnar
T. Long-term outcome of
transctheter patent ductus arteriosus closure using Amplatzer duct occluders. Am
Heart J 2006; 151e7-755.e10.