ABSTRACT
Objective: To describe various types of congenital renal
anomalies incidentally detected during routine DMSA scan in children with
urinary tract infection, and to compare the incidence of scarring in patients
with and without renal anomalies.
Methods: This study included 400 subjects (138 boys and 262
girls), age range (one month to 15 years / Mean= 5.6 years). In the period between
May to December 2009, children were referred to Nuclear Medicine Center for Tc
99m DMSA scan to rule out renal scarring. Congenital anomalies appearance,
scarring and function of kidneys were documented. Pearson correlation was used
in statistical analysis and P<0.05 was considered significant.
Results: There were 55
cases of congenital kidney anomalies in our study (13.75%), within 29 boys and
26 girls. The most common congenital anomaly was single kidney seen in 17 cases
(4.25%). Renal scarring was detected in 31.25% of total cases (125 cases out of
400 cases), 30.9% of congenital anomalous kidneys (17 out of 55 cases), and in
31.3% of non-anomalous kidneys (108 out of 345 cases).
Conclusion: Congenital renal anomalies are not uncommon. Tc 99m
DMSA scan is an adequate imaging modality to detect these anomalies and assess
renal scarring. Patients with congenital anomalies did not show an increase in
renal scarring compared to non-anomalous kidneys.
Key words: Tc99m- DMSA, Congenital renal anomalies, Scarring, Renal
scan.
JRMS
June 2011; 18(2): 36-42
Introduction
Renal tract malformations are a
clinically challenging collection of entities because of their diversity, and
the fact that these disorders can present both before and after birth. The most
severe anomalies can be devastating, resulting in neonatal renal failure and
death. At the other extreme, some of the milder, more common anomalies can have
a benign course. Each patient with a renal tract malformation, therefore, needs
an individualized clinical approach, which might require diverse
investigational modalities, most important of which is Tc99m DMSA cortical scan.(1)
Being common in children, about 1-2% of boys and 3-7%
of girls experience at least one episode of urinary tract infection (UTI)
before the age of 11 years. Assessment of renal parenchymal damage resulting
from acute or chronic renal infection is the primary indication for
radionuclide imaging with Tc-99m DMSA.(2) In addition, this
technique allows congenital anomalies and permanent renal scarring to be
identified. The aim of this study was to assess various types of congenital
renal anomalies detected during routine DMSA scan in children with UTI by describing
the appearance of such anomalies. Furthermore, we compared the incidence of
scarring in patients with and without renal anomalies.(2-4)
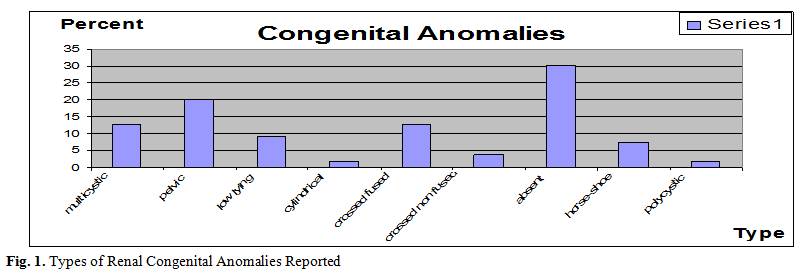
Table I. Percentage of different anomalies and incidence of
scarring
|
Congenital Anomaly
|
Total
|
Scarred
|
% of scarring
|
% from total cases
|
% from anomalies
|
|
Absent
|
17
|
0
|
0
|
4.25
|
30.2
|
|
Pelvic
|
11
|
9
|
81.8
|
2.75
|
20.1
|
|
Crossed Fused
|
7
|
4
|
57
|
1.75
|
12.8
|
|
Multicystic
|
7
|
0
|
0
|
1.75
|
12.8
|
|
Low lying
|
5
|
1
|
20
|
1.25
|
9.2
|
|
Horse-Shoe
|
4
|
2
|
50
|
1
|
7.4
|
|
Crossed Non Fused
|
2
|
1
|
50
|
0.5
|
3.7
|
|
Cylindrical
|
1
|
0
|
0
|
0.25
|
1.9
|
|
Polycystic
|
1
|
0
|
0
|
0.25
|
1.9
|
Methods
This study included 400 children (138 boys and 262
girls), aged one month to 15 years (Mean 5.6 years). All children had UTI, and
were referred for the Nuclear Medicine Center at King Hussein
Medical Center
for DMSA renal scan in the period from May to December 2009, to assess for
possible renal scarring. All patients had DMSA scan conducted two to three hours
after intravenous injection of Tc99m- DMSA radiopharmaceutical, with calculated
dose according to body weight. A posterior, anterior and two posterior oblique
images of the kidneys were acquired, with the patient supine on a dedicated
dual head Millennium Gamma Camera.
The renal scintigraphic patterns were independently
interpreted by two senior nuclear-medicine physicians, and the criteria used
for the interpretation of the images regarding
renal scars and congenital anomalies did not change during the period of the
investigation. A kidney with normal size, regular shape and a tracer uptake
that appeared to be homogenous was considered as normal. Single or multiple
wedge shaped cortical defects, focal or diffuse photopenic patterns in one
kidney with or without contraction and loss of volume in the involved cortex
were considered as abnormal and indicating scarring.(5,6) The
normal position of the kidney was taken into consideration for assessment of
ectopia.
Cases were classified as normal kidneys with regard to
position and shape irrespective to scarring, and kidneys with otherwise
congenital anomalies noted, namely: single kidney, pelvic kidney, multicystic
kidney, crossed fused ectopia, low lying kidneys, horse-shoe kidney, crossed
non-fused ectopia, cylindrical kidney and polycystic kidneys.
Pearson correlation
was used to determine the statistical significance of the relationships between
variables studied: scarring in patient with or without renal congenital
anomalies. The Pearson correlation was determined (r value), and a P value
below 0.05 was considered statistically significant.
Results
There were 55 cases of congenital renal anomalies in
our study (13.75%), within 29 boys and 26 girls. The most common congenital
anomaly encountered was single kidney which appeared in 17 cases accounting for
4.25% of total cases (30.2% of anomalies). The second most common was pelvic
kidney, seen in 11 accounting for 2.75% incidence from total cases (20.1 % of
congenital anomalies). Multicystic
kidney and crossed fused ectopia had the same percent with 7 cases each accounting
for the same percent: 1.75% each.
Low lying kidneys were considered in cases with kidneys lying lower than
the contralateral but still not pelvic, these accounted for 1.25 % seen in 5
cases. Horse-shoe kidney accounted for 1% (4 cases). Kidneys crossing the
midline and not fused with the other kidney (crossed non-fused ectopia) was
seen in 0.5% of cases (2 cases). One case of cylindrical kidney (0.25%) and one
case of polycystic kidneys (0.25%). (Fig. 1, Table I)
The other factor studied other than anomalies of the
kidneys, was presence of renal scarring. This was considered scintigraphically
as any size of cortical wedge defect, diffuse thinning of cortex or smaller
size shrunken kidney.(5,6) These findings were approved by
two senior nuclear medicine specialists in the department. Renal scarring was
detected in 31.3% of non-anomalous kidneys (108 of the 345 cases), and 30.9% of
congenital renal anomalies (17 of the 55 cases). The Pearson product-moment correlation
coefficient was used to determine the relation between the two groups. Pearson
Correlation was (r = 0.075) indicating insignificant statistical difference in
the incidence of scarring between the two groups with an equivalent P value = 0.133.
Discussion
UTI is common in
children. About 1-2% of boys and 3-7% of girls experience at least one episode
of UTI before the age of 11 years.(6,7) Assessment of renal
parenchymal damage resulting from acute or chronic renal infection is the
primary indication for radionuclide imaging with Tc-99m DMSA.(1,2,4)
It is a more sensitive modality compared to IVU and ultrasound in the
evaluation and follow up of kidneys at risk for scarring in children, and
allows congenital anomalies to be identified.(2,3,7) Tc-99m
DMSA is taken up specifically in the tubular cells of the renal cortex and
facilitates assessment of function and identification of aberrantly located
kidneys, and the assessment of outcome of urinary tract infection or VUR, namely
cortical scarring.(8) Although some authors mention the low
yield of positive results on the management in children more than one year of
age.(9,10)
Unilateral renal agenesis is not uncommon and usually
is accompanied by ureteral agenesis with absence of the ipsilateral trigone and
ureteral orifice. No treatment
is necessary; compensatory
hypertrophy
of the solitary kidney maintains normal renal function.(11–13)
A similar entity, renal aplasia, is evident by absent activity on DMSA scan
with the presence of renal parenchyma by ultrasound.(3,10,14)
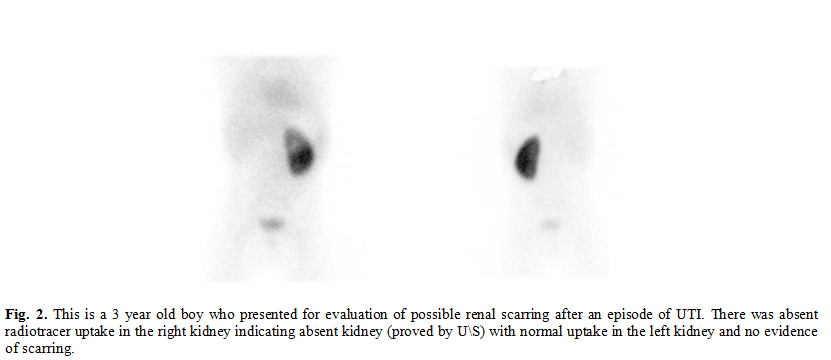
In the 400 cases we reviewed, 17 cases of congenitally absent kidney were
found, non of which showed any cortical scarring in the contralateral kidney.
This finding encouraged us to hypothesize that a single kidney as isolated
congenital anomaly doesn’t increase the risk of cortical scarring. (Fig. 2).
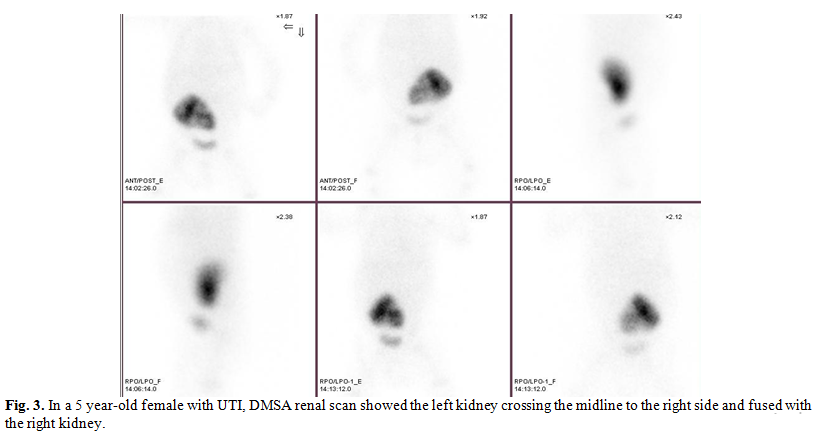
Embryologic
development of crossed renal ectopia has not been clearly determined but many
theories have been offered to explain this congenital anomaly. It is suggested
that mechanical factors are of primary importance in ectopia without fusion.
Being more frequent in males (M/F = 1.4/1), crossed renal ectopia is two to
three times more common on the right than on the left.(11,15)
In our study of the 9 cases of crossed
ectopia (fused and non-fused ) 7 cases were situated on the right and only 2
cases on the left side. The condition is generally diagnosed in the third
decade. Crossed fused ectopia appear to be more prevalent in our study
population compared to crossed non-fused ectopia (12.8% of congenital anomalies
compared to 3.7% for crossed non-fused ectopia), which is also the case in
other reviews.( 15,16) ( Fig. 3)
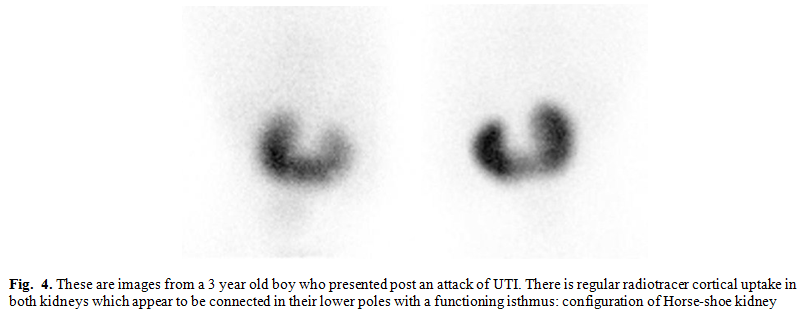
Horse-shoe kidney
occurs when renal parenchyma on each side of the vertebral column is joined at
the corresponding (usually lower) poles; an isthmus of renal parenchyma or
fibrous tissue joins at the midline (Fig. 4). The ureters course medially and
anteriorly over this isthmus and generally drain well.(11,17)
In our review we had 7 cases,
contributing to 7.4% of total renal anomalies. Of these non showed any renal
scars, suggesting no clinical relevance between this entity and incidence of
scarring.
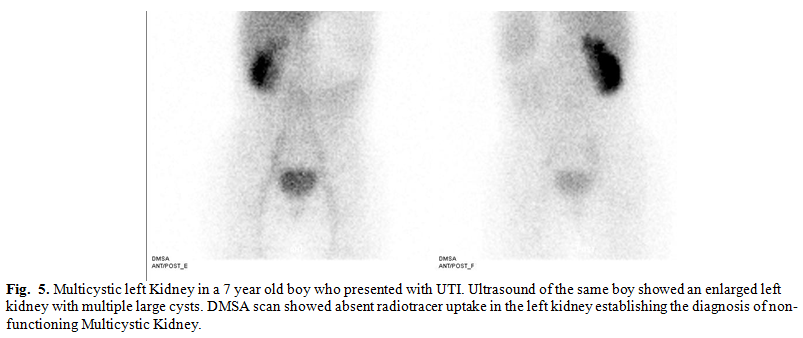
Multicystic kidney was
observed in 7 cases, contributing to 12.8% of renal anomalies in our study population
(Fig. 5). In this condition, a nonfunctioning renal unit consists of non-communicating
cysts with intervening solid tissue composed of fibrosis, primitive tubules,
and foci of cartilage. Usually, ureteral atresia is also present. Uncommonly,
the kidney develops tumors or infection, and hypertension may develop. Most
experts recommend observation, although some advocate removing these kidneys.(3,11,18
)
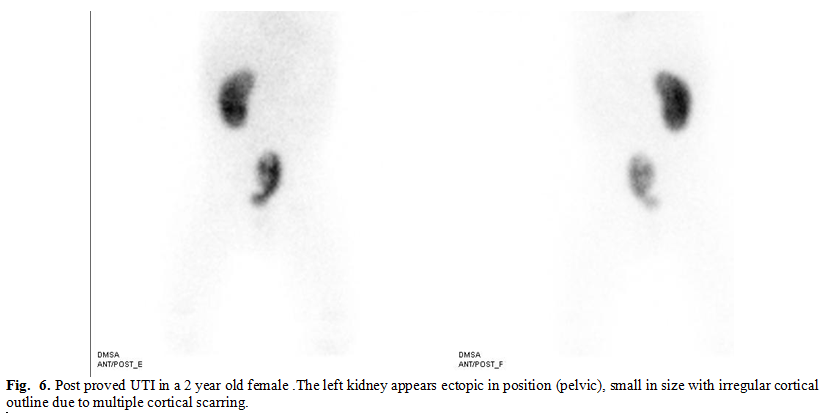
Renal ectopia,
abnormal renal location, usually results when a kidney fails to ascend from its
origin in the true pelvis (Fig. 6); a rare exception occurs with a superiorly
ascended (thoracic) kidney.(19) Pelvic ectopia increases the
incidence of
Table
II.
Frequency of scarring in anomalous kidneys and normal situated
(non-anomalous) kidneys, and percent of scarring taking into account the total
number and the number of each group
|
|
Number of cases
|
% from total
N=400
|
%
from specific group
|
|
Total
Scarred from non-anomalous kidneys
|
108
|
27.0
|
31.3
(from non-anomalous kidneys: N=345)
|
|
Scarred
anomalous kidneys
|
17
|
4.25
|
30.9
(from anomalous kidneys: N=55)
|
|
Total
|
125
|
31.25
|
|
ureteropelvic junction
obstruction, vesicoureteral reflux, and multicystic renal dysplasia.
Obstruction is corrected surgically. Severe reflux can be corrected surgically
when indicated (if causing hypertension, recurrent infections, or renal growth
retardation).(1,3) The incidence of renal scarring is
increased in pelvic kidney compared to other renal anomalies. In our study
pelvic kidneys accounted for 20.1% of total anomalies (11 cases), of these
81.8% were scarred (9 cases). We noticed the right kidney to be more liable to
failure of ascendance as 8 cases of the 11 pelvic kidneys affected the right side.
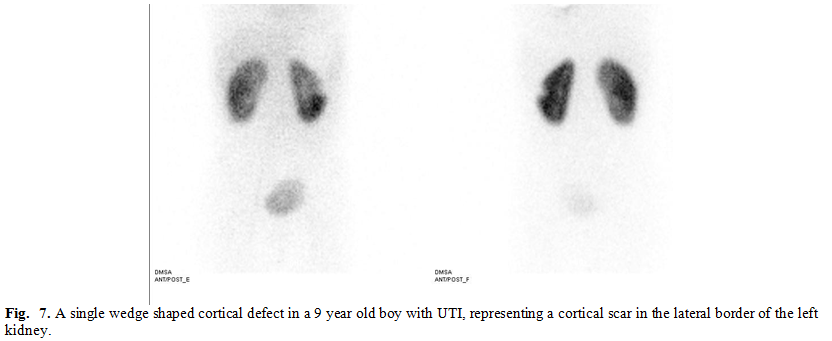
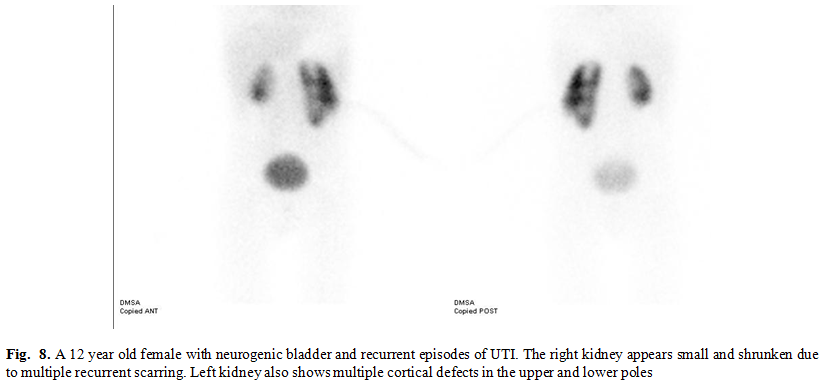
During reviewing the
400 cases, scarring (evident by single or multiple wedge shaped cortical
defect, diffuse cortical thinning or shrunken small kidney) was noted in 31.25%
of all cases (125 cases), 31.3% of normal situated kidneys (non- anomalous)
(108 cases) and 30.9 % of congenital anomalies (17 cases) (Fig. 7, 8). These
results appear to be statistically insignificant in regard to renal scarring
incidence. Although some congenital anomalies as single entities had increased
incidence of scarring, taking congenital anomalies as an entire assembly was not
associated with increased incidence of parenchymal insult and cortical scarring
in our study. (Table II)
Conclusion
Congenital renal anomalies
are usually diagnosed when other disease states are being investigated during
DMSA scan, namely chronic or acute urinary tract infections. This scan can be
helpful in assessment and follow-up of kidney functions as well as assessment
of renal scarring. Patients with congenital anomalies, as entire assembly, did
not show any increase in incidence of renal scarring compared to normal kidneys.(20)
Acknowledgement
We would like to give
special thanks and gratitude to Brig. Gen. Issa Hazza MD, consultant pediatric
nephrologist at the Royal Medical Services who helped us with collecting these
cases and guided us with his valuable knowledge.
References
1.Dave
S, Khoury A. Diagnostic approach
to reflux in 2007. Advances in Urology 2008; 2008:367320.
2.Ayit E S, Yilmaz M, Yorulmaz I, et
al. Tc-99m DMSA scintigraphy
in recurrent urinary tract infection in children. J Med Res 2000; (18):17-21
3.Kerecuk
L, Schreuder MF, Woolf A S. Renal tract malformations:
perspectives for nephrologists. Nature Clinical
Practice Nephrology 2008; (4): 312-325.
4.Pohl HG, Belman AB. The “Top-Down” Approach to the evaluation of children
with febrile urinary tract infection. Advances in Urology 2009;
2009:
783409.
5.Itoh K, Yamashita T, Tsukamoyo E,
Nonomura K, et al. Qualitative
and quantitative evaluation of renal parenchymal damage by Tc 99m-DMSA planar
and SPECT Scintigraphy. Annals of Nuclear Medicine 1995; 9 (1): 23-28.
6.Clarke SEM, Smellie JM, Prescod N. Technetium-99m-DMSA studies in pediatric urinary
tract infection. The Journal Of Nuclear Medicine 1996; 37(5): 823-828.
7.Ataei
N, Safaian B, Madani A, et al. The importance of Tc 99m- DMSA renal scintigraphy in evaluation of
renal lesions in children with acute pyelonephritis. Acta Medica Iranica 2008;
46 (5): 399-404.
8.Siomou E, Giapros V, Fotopoulos A, et al. Implications of 99mTc-DMSA
scintigraphy performed during urinary tract infection in neonates. Pediatrics 2009; 124(3):
881-887.
9.Deshpande PV, Jones KV. An audit of RCP guidelines on DMSA scanning after urinary tract infection. Arch Dis Child 2001; 84: 324-327.
10. Ahmadzadeh A, Askarpour S. Association of urinary tract abnormalities in children
with first urinary tract infection. Pak J Med Sci January - 2007; 23 (1):
88-91.
11.Vijayakumar V,
Address:
Jackson
Mississippi
USA
Nishino T.K. Congenital pediatric anomalies: a collection of
abdominal scintigraphy findings: an imaging atlas. Internet Journal of
Nuclear Medicine 2008; 5(1).
12. Scott JES. Fetal, perinatal and infant death with congenital renal anomaly. Archives of Disease in Childhood 2002; 87:114-17
13. Hiraoka
M, Tsukahara M, Ohshima Y, et al. Renal aplasia is the predominant
cause of congenital solitary kidneys. Kidney International 2002;
61: 1840–1844.
14. McPherson E. Renal anomalies in families of individuals with congenital solitary kidney. Genet Med 2007 May; 9(5):298-302
15. Nursal GN, Buyukdereli G. Unfused renal ectopia: a rare form of congenital renal
anomaly. Annals of Nuclear Medicine 2005; 19(6): 507–510.
16. Ravi S, Truong M X, Rossleigh M A, et al. Renal scintigraphy unraveled the diagnostic dilemma of
antenatal hydronephrotic solitary kidney-crossed renal ectopia. Clinical Nuclear Medicine 2005; 30(9): 621-22.
17.
Volkan B, Ceylan E, Özgen KP.
Radionuclide imaging of rare congenital renal fusion anomalies. Clinical
Nuclear Medicine 2003; 28 (3): 204-07.
18. Merck Manual Professional. Renal anomalies: Congenital renal and genitourinary anomalies.
Merck Manual of Diagnosis and Therapy, Last full review/revision November
2005.
19. Srinivasan
V, Balasubraman N, Hariprasad B, et al. A roentgenological
evaluation of thoracic kidney. Indian J Chest Dis Allied Sci 2003; 45:
55-57.
20.
Cherchi SS, Ravani
P, Corbani V, et al. Renal
outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney International 2009;
76: 528–33.