ABSTRACT
Objectives: Calcium carbonate/magnesium carbonate (CaCO3/MgCO3) combination chewable tablets may be an attractive alternative phosphate binder to calcium carbonate (CaCO3) tablets with potential advantages of synergistic phosphate binding effect. The aim of this study is to evaluate the differences between the two phosphate binders on 42 hemodialysis patients who are taking H2-Blockers in terms of serum PO4-3 and Mg+2levels.
Methods: Randomized, controlled, open label study was conducted at the renal /hemodialysis unit of King Hussein Medical Center for six weeks (On May 2018). After the study was approved, patients who met the inclusion criteria were enrolled and were randomly allocated into an interventional group (Group I) and a control group (Group II). Follow-up continued for 6 weeks. The collected data were statistically analyzed using the independent t-test.
Results: A total of 37 hemodialysis patients were finally included in this study. The mean age was 40.81±2.31 years, 17 subjects (45.95%) were males, and 20 subjects (54.05%) were females. Despite serum PO4-3 level decreased significantly in group I by -0.39±0.99 mg/dl after replacing CaCO3 tablets by CaCO3/MgCO3 in equivalent dose, the differences between two groups were statistically insignificant (-0.094±0.213). But when taking into consideration the incremental cost-effectiveness, the CaCO3/MgCO3 tablets are more cost effective by magnitude of 27.766 JD per week for every 1 mg/dl. Serum magnesium level increased significantly within group I and between the two groups by +0.55±0.36 mg/dl and +0.430±0.089 mg/dl respectively. Neither serum cCa+2 nor cCa+2 ×PO4 -3 products were changed significantly either among or between the two groups.
Conclusion: No significant difference was detected between Ca-Mg carbonate chewable tablets and the less expensive Ca carbonate tablets in reducing serum phosphate levels in hemodialysis patients on H2-R blockers.
Key words: Calcium carbonate, Hemodialysis, Magnesium carbonate, Phosphate binders.
JRMS April 2019; 26(1): 43-49/ DOI: 10.12816/0052898
Introduction
Calcium and phosphate homeostasis is regulated by the kidneys, GIT and bones. As kidney function deteriorates, phosphate retention increased and activation of Cholecalciferol to 1-OH-cholecalciferol are decreased (1,2). Patients with chronic kidney disease (CKD) experience hypergastrinemia due to decrease clearance of gastrin and increase density of G cells that secret gastrin, hence the prescription of histamine 2 blockers (H2-Blockers) or proton pump inhibitors (PPIs) is usually recommended to CKD patients (3) (4) .CaCO3 is routinely used as a phosphate binder in CKD patient, however there are multiple pharmacological therapies as phosphate binder; Rennie®(5) which is calcium carbonate/magnesium carbonate (CaCO3/MgCO3)combination chewable tablet contains 80 mg of magnesium carbonate (MgCO3) and 680 mg of calcium carbonate (CaCO3) per tablet.CaCO3 tablets are cheaper than Ca-Mg carbonate chewable tablets.
However, the dissolution of calcium carbonate tablets is pH dependent and is decreased when stomach pH is increased by co-administration of H2R-blockers while the dissolution of Ca-Mg chewable tablets is less affected by alteration of stomach pH (6). Using Mg carbonate alone no better at lowering serum phosphate levels, and was associated with more dose-limiting side effects(7) MgCO3 is an effective and inexpensive agent to control serum phosphate levels in hemodialysis patients. Gastrointestinal disorders due to its use were minor, while its administration in combination with a low dialysate magnesium concentration reduces the risk of severe hypermagnesemia. Patients treated with MgCO3 had, an optimum regulation of cCa+2×PO4 -3product, relatively low serum calcium and no episodes of hypercalcemia,(8) chronic mild hypermagnesemia may decrease PTH synthesis and/or secretion, and could be a very useful adjunctive therapy in alleviating complications of CKD-MBD. In this way it can offer viable alternatives to the combination of calcium as a phosphate binder and vitamin D therapy; a strategy that may lead to frequent hypercalcemia episodes, calcium accumulation and potentially harmful cardiovascular consequences.(9)
Methods
This randomized, controlled, open label study was conducted at renal /hemodialysis unit of King Hussein Medical Hospital (KHMC) for six weeks. After the approving from the IRB committees at the Jordanian Royal Medical Services, patients in the renal /hemodialysis unit of KHMC who met the inclusion and didn’t meet the exclusion criteria as described in (Figure1). patients enrolled in this study after they accepted to participate in this randomized, controlled, open label study, they were randomly allocated into interventional group (Group I) and control group (Group II). Blood samples were drawn from the HD participants before HD session and heparin infusions were started. The tests of serum PO4 -3, Serum Ca+2, serum Mg+2, serum K+, blood glucose, SCr, and BUN levels were performed within 1-2 hours after the unheparizined blood was immediately separated by centrifugation in the KHMC chemistry laboratories. Serum cCa+2, cCa+2×PO4 -3 product and PO4-3 × Mg+2/ cCa+2 ratios were calculated manually. All previous tests were measured on weekly basis for the first 2 weeks and then every other week. This study used a dialysate concentration of Mg+2 0.73 mg/dl (0.6 meq/l) for the MgCO3 group and 1.16 mg/dl (0.96 meq/l) for the CaCO3 group, whereas dialysate Ca concentration was 6 mg/dl (3 meq/l) for both groups.
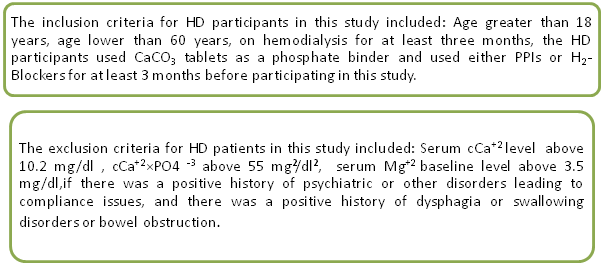
Fig 1.Inclusion and exclusion criteria for hemodialysis patients.
All possible retrospective data for Group I and Group II were collected before the study period was started. The retrospective data that we collected (data of three months ago), included the last three values of serum PO4 -3 levels, serum Ca+2 levels, serum albumin levels, serum Mg+2 levels, and number of CaCO3 tablets that were used as phosphate binder per day (n1). After retrospective data were completed, the two studied groups were followed for 6 weeks in which the following outcomes were measured and assessed in the following basis:
• Serum PO4 -3 level, serum Ca+2 level , serum albumin level, serum cCa+2 level, cCa+2×PO4 -³ product, and serum Mg+2 level were measured on weekly basis for the first 2 weeks and then every other week
• n1, number of CaCO3/MgCO3 combination chewable tablets (Rennie®) per day that were used as phosphate binder (n2), and the ∑ phosphate binder cost per week were assessed on weekly basis.
In the interventional prospective follow-up, the CaCO3 tablets in Group I were replaced totally by CaCO3/MgCO3 combination chewable tablets (Rennie®) without a washout period (maximum 6 tablets per day), in which each 1 tablet of CaCO3 1250 mg was replaced by 2 tablets of CaCO3/MgCO3 combination 680 mg/80 mg (Rennie®), while keeping all other medications without any change. During the follow-up phase, the CaCO3 tablets replacement by CaCO3/MgCO3 combination chewable tablets (Rennie®) in interventional group was assessed on weekly basis and If serum Mg+2 level was ≥3.5 mg/dl and persisted for 1 week or serum Mg+2 level was ≥4.5 mg/dl we dropped-out the HD participant from our study. The CaCO3 tablets in Group II were kept without any change in the prospective follow-up phase.
Blood samples were drawn from the HD participants before HD session and heparin infusions were started. The tests of serum PO4 -3, Serum Ca+2, serum albumin, and serum Mg+2 were performed within 1-2 hours after the unheparizined blood was immediately separated by centrifugation in the KHMH chemistry laboratories. Serum cCa+2 and cCa+2×PO4 -3 products were calculated manually. The values of number of PO4-3 binder(s) tablet(s)/ day were obtained directly from HD participants. The Σ cost per week was calculated manually.
After follow-up part of this open label randomized controlled trial was finished at the end of 6 weeks. The collected data of each outcome in the different two studied groups were analyzed using independent t-test.
Results
The recruitment, randomization, and dropout processes of all 142 eligible HD participantsand the medical and medication history of the study candidates in each group of the two studied groups are summarized in (Figures 2-3).All demographic characteristics of 37 HD participants in the two studied groups are summarized in (Tables I-II).
Fig 2: Recruitment, randomization, and dropout processes scheme
Fig 3: Patient's medical and medications history of the HD participant patients presented as (percentage).
Table I: Demographic characteristics of the two groups.
|
|
Group I N=19
Mean±SD
|
Group II N=18
Mean±SD
|
Total N=37
Mean±SD
|
P- Value
|
Sig
|
|
Age (years)
|
39.47±2.47
|
43.21±2.01
|
40.81±2.31
|
0.349
|
NS
|
|
Sex
|
Male (%)
|
7 males
(41.18%)
|
10
males
(58.82%)
|
17
males (45.9%)
|
0.438
|
NS
|
|
Female (%)
|
12
females
(60 %)
|
8
females
(40 %)
|
20
females (54.1 %)
|
|
BMI (kg/m²)
|
22.21±0.043
|
26.51±0.039
|
24.79±0.043
|
0.897
|
NS
|
Data are presented as Mean±SDor as percentage by using Independent t-test (at p-value< 0.05)
S*: Significant -NS: Non significant-BMI: Body mass index -KHMH: King Hussein Medical Hospital
Table II: Other demographic characteristics of the two studied groups.
|
Characteristics
|
Group I
N=19
Mean±
SD
|
Group II
N=18
Mean±
SD
|
Total
N=37
Mean±
SD
|
P-Value
|
Sig
|
|
Duration of dialysis (months)
|
97.68±15.338
|
93.44±11.628
|
94.03±7.496
|
0.036
|
S*
|
|
Duration of using CaCO3 tab
as phosphate binder (months)
|
97.68±15.338
|
93.44±11.628
|
94.03±7.496
|
0.036
|
S*
|
|
Duration of using
H2-Blockers (months)
|
75.47±11.136
|
90.33±11.998
|
81.38±6.945
|
0.315
|
NS
|
|
HD duration per session (hours)
|
4.18±0.109
|
4.08±0.061
|
4.12±0.047
|
0.158
|
NS
|
|
HD frequency
Per week (%)
|
1*per week
|
1
patient (5.3%)
|
0
patient
(0%)
|
1
patient (2.7%)
|
0.313
|
NS
|
|
2*per week
|
5
patients (26.3%)
|
5
patients (27.8%)
|
10
patients
(27%)
|
|
3*per week
|
13
patients (68.4%)
|
13
patients (72.2%)
|
26
patients (70.3%)
|
|
4* per week
|
0
patient
(0%)
|
0
patients (0%)
|
0 patient
(0%)
|
A dependent T-test was conducted to determine whether there were significant differences within the two studied groups and independent t-test in parameters that are summarized in (Table III).
Table III: Serum PO4‾³ levels and the related variables values differences between the comparative groups.
|
Comparative
Groups
Affective
Variables
|
Group I after
Versus
Group I before
|
Group II after
Versus
Group II
before
|
Group I
Versus
Group II
|
|
Serum PO4‾³
level (mg/dl)
Mean
difference±SD (Sig)
|
-0.39±0.99
(S*)
|
-0.29±0.35
(S*)
|
-0.094±0.213
(NS)
|
|
Serum cCa+²
level (mg/dl)
Mean
difference±SD (Sig)
|
+0.08±0.48
(NS)
|
+0.23±0.39
(S*)
|
(NS)
|
|
cCa+2×PO4-3 (mg²/dl²)
Mean
difference±SD (Sig)
|
-3.19±8.09
(NS)
|
-1.52±3.33
(NS)
|
-1.663±1.789
(NS)
|
|
Serum Mg+²
level
Mean
difference±SD (Sig)
|
+0.55±0.36
(S*)
|
+0.12±0.17
(S*)
|
+0.430±0.089
(S*)
|
|
n1
(tablet/day)
Mean
difference±SD (Sig)
|
-2.433±0.667
(S*)
|
-0.083±0.354
(NS)
|
-2.35±0.15
(S*)
|
|
n2 (tablet/day)
Mean
difference±SD (Sig)
|
+4.592±1.036
(S*)
|
0
|
+4.59±0.24
(S*)
|
|
CaCO3
(mg/day)
Mean
difference±SD (Sig)
|
+81.84±608.84
(NS)
|
0.000±0.000
(NS)
|
(NS)
|
|
Σ Cost per
week
Mean
difference±SD (Sig)
|
-2.37±0.75
(S*)
|
+0.24±2.54
(NS)
|
-2.610±0.532
(S*)
|
|
MgCO3
(mg/day)
Mean
difference±SD (Sig)
|
+367.368±82.879
(S*)
|
0
|
+367.37±19.52
(S*)
|
Data are presented as Mean difference ±SD (at p-value< 0.05)
- S*: Significant -NS: Non significant
- n1 (tablet/day): Number of CaCO3 tablets per day that were used as phosphate binder.
-n2 (tablet/day): Number of CaCO3/MgCO3combination chewable tablets per day.
-n4 (tablet/week): Number of H2-Blocker tablets per week.
-CaCO3 (mg/day): Amount of CaCO3 in mg per day from either CaCO3 tablets that were used as phosphate binder or from CaCO3/MgCO3combination chewable tablets or from both.
CaCO3/MgCO3combination chewable tablets.
Σ Cost per week: Total cost per week in $/week.
Despite serum PO4-3 level decreased significantly in group I by -0.39±0.99 mg/dl after replacing CaCO3 tablets by CaCO3/MgCO3 in equivalent dose, the differences between group I and group II were insignificant (-0.094±0.213). But with a significant lower Σ Cost per week with mean differences of -3.68±0.75 $/week. In contrast to serum Mg+² level, which increased significantly within group I and between the two groups by +0.55±0.36 mg/dl and +0.430±0.089 mg/dl respectively. Neither serum cCa+2 nor cCa+2×PO4 -3 products were changed significantly either among or between the two groups.
Discussion
There were some studies addressing the adverse effect of H2-Blockers when combined with phosphate binders especially the most commonly used CaCO3. For example, Tan,et aland colleagues conclude that ranitidine has a significant adverse effect on binding of CaCO3to phosphate in patients with renal failure, serumPO4-3 levels were significantly higher during the ranitidine than the placebo phase (5.51±1.33 mg/dl versus 4.92±1.52 mg/dl; p<0.001) and there was no significant change in serum c Ca+2 (10). These results were explained depending on the pH effects on dissolution and kinetic binding of CaCO3tablet; the acidity is best for solubility (11) .In contrast CaCO3/MgCO3 combination chewable tablet has no problem in dissolution after either chewing or sucking so that, there is no drug interaction with H2-Blockers on the dissolution step (rate-limiting step), in addition to that, the higher pH in stomach that is created by H2-Blockers will potentiate the second step of phosphate binding scenario (binding of Ca+2 and Mg+2 to the PO4‾³ in the GIT). In our study, we conclude that there was no significant differences in serum PO4-3and cCa+2when the H2-Blockers are added to the phosphate binders in both groups may be due improved compliance to CaCO3 tablet intake in group II as described by significant change in serum PO4-3 (-0.29±0.35). Despite this insignificant differences, the CaCO3/MgCO3 combination has a total lower cost per week (-3.68±0.75) when compare with CaCO3, which give the advantage of CaCO3/MgCO3 combination chewable tablets. As expected, the serum Mg+² level was increased significantly in this study (+0.430±0.089) as in Delmez, et alcontrolled cross over study when adjusted dose of CaCO3/MgCO3 combination tablets replaced CaCO3 tablets monotherapy group to achieve target serum phosphate concentrations of <6.0 mg/dL and serum calcium concentrations of 9.5–10.5 mg/dl. Serum levels of PO4-3,cCa+2 were similar in the both phases,serum Mg+2 was also similar in both phase due to using 1/3 dialysate Mg+2 in the CaCO3/MgCO3 combination tablets phase which means CaCO3/MgCO3 combination tablets can increase serum Mg+2 level (12) .
Limitations of the study:
The following limitations were present in this study:
-There was no washout period in this study.
-The sample size was small and should be increased.
-Cost effectiveness is not constant but variable factor from time to time.
Conclusions
No significant difference was detected between Ca-Mg carbonate chewable tablets and the less expensive Ca carbonate tablets in reducing serum phosphate levels in hemodialysis patients on H2-R blockers.
Acknowledgment:
We thank Dr.Salah Abu Ruz,Prof, The University of Jordan and Dr.Izzat Alawwa,Nephrologist, Jordan University Hospital, for their assistance and comments on an earlier version of the manuscript.
REFERENCES
1-Drueke T, Foley RN, et al. Improving outcomes in chronic kidney disease.Kidney International Supplement 2006; 105:S1–S4.
2-Fraser D, Jones G, Kooh SW, et al. Calcium and phosphate metabolism. Fundamentals of clinical chemistry3rd ed.Philadelphia, Pa: WB Saunders 1987; 705-728.
3-Crivelli O, Pera A, Lombardo L, Vernero S, Varetto H, Fruttero B, et al. AntralG and D cell counts in chronic renal failure.Scandinavian Journal of Gastroenterology 1979; 14:327–331.
4-Carlei F, Caruso U, Lezoche E, Ruscitto G, Laukie P , Casciani U et al. Hyperplasia of antral G cells in uremic patients. Digestion 1984; 29:26–30.
5-Sheikh MS, Maguire JA,Emmett M et al. Reduction of dietary phosphorus absorption by phosphorus binders: a theoretical, in vitro, and in vivo study. Journal of Clinical Investigations 1989; 83: 66-73.
6-Clarkson EM, McDonald SJ, De Wardener HE et al. The effect of a high intake of calcium carbonate in normal subjects and patients with chronic renal failure. Clinical Science1966; 130: 425-438.
7-Baigalmaa Evsanaa, Irene Liu, Babak Aliazardeh, Sara Mahdavi, Gursarn Bajwa,Jerry Gula,Michelle Tam, Elena Sze,Janet M. Roscoe,Paul Y. Tam,and Tabo Sikaneta et al.MgCaCO3versus CaCO3in peritoneal dialysis patient – A cross-over pilot trial. Peritoneal Dialysis International, Vol. 35, pp. 31–34.
8-Ioannis P. Tzanakis Æ Antonia N. Papadaki Æ Mingxin Wei Æ Stella Kagia ÆVlassios V. Spadidakis Æ Nikolaos E. Kallivretakis Æ Dimitrios G. Oreopoulos et al.Magnesium carbonate for phosphate control in patients on hemodialysis. A randomized controlled trial. Int Urol Nephrol (2008) 40:193–201
9-Mehmet Kanbay , David Goldsmith , Mehtap Erkmen Uyar , Faruk Turgut ,Adrian Covic et al.Magnesium in Chronic Kidney Disease:Challenges and Opportunities. Blood Purif 2010;29:280–292
10-Tan CC, Harden PN, Rodger RS, Rowe PA, Spooner RJ, Junor JD , Briggs JD et al. (1996),Ranitidine reduces phosphate binding in dialysis patients receiving calcium carbonate.Renal Unit and Department of Biochemistry 1996; 11(5): 851-853.
11-Carr C, Shangraw R et al. Nutritional and pharmaceutical aspects of calcium supplementation. Journal of American Pharmacists Association 2005; 27: 49–50, 54– 57.
12-Delmez JA, Kelber J, Norword KY, Giles KS,Slatopolsky E et al. Magnesium carbonate as a phosphorus binder: a prospective, controlled, crossover study.Kidney International 1996; 49(1): 163–167.