ABSTRACT
Objective: With the LCZ696 (sacubutril/valsartan) being a new add-on treatment to patients with congestive heart failure and reduced ejection fraction, this study aims to show the effects of LCZ696 on ejection fraction (first reported) and the quality of life of the Palestinian population with heart failure.
Methods: The first 102 patients who were started on this medication were examined. In addition, all demographic data were tabulated, and all follow-up visits were analyzed. The quality of life based on the New York Heart Association (NYHA) classification was recorded. All hospital admissions, deaths, labs, and echocardiograms were recorded. The length of study was 6–12 months (mean of nine months), and all patients, signed an informed consent form allowing for their charts to be reviewed.
Results: The LCZ696 has been studied for over a period of 6–12 months on 102 patients with heart failure and reduced ejection fraction. Measurement of ejection fraction was measured by standard protocol before, during, and after initiating LCZ696. Records showed a significant improvement in the left ventricular EF and in their NYHA classification of heart failure.
Conclusion: Despite its limitations, we think that LCZ696 (sacubutril/valsartan) has a very promising outcome on the quality of life and the ejection fraction of a group of patients with congestive heart failure and reduced ejection fraction. There were statistical improvements on the quality of life as well as in the ejection fraction (first reported) with an acceptable safety margin. However, such effect needs to be replicated in other prospective and retrospective studies.
Keywords: Congestive heart failure, LCZ696 (sacubutril/valsartan), Palestine, Quality of life
JRMS August 2019; 26(2):73-78/ DOI: 10.12816/0053294
Introduction
Congestive heart failure (CHF) is a devastating chronic problem. It accounts for nearly one million admissions to US hospitals per year. Despite the available therapy (standard of care), mortality still approaches 50% at 5 years, (1) and it results in an enormous amount of health burden on any community.(2)
Recently, a new medication, LCZ696 (sacubutril/valsartan), was offered as a newer therapy for such patients. A major trial (the paradigm HF trial) showed not only an improved quality of life but also a 20% reduction in mortality, which led to termination of the trial before its term.(1)
For the first time, we studied the effect of LCZ696 on Palestinian patients who were started on this new medication and tested its effect on the progression of heart failure in the listed study group based on the New York Heart Association (NYHA) classification of heart failure and using the echocardiographic evidence of improvement in the left ventricular ejection fraction (EF), which has not been reported.
Methods
A total of 102 patients were included in this retrospective chart analysis. All these patients were started on LCZ696 prior to the study because of clinical reasons based on discretion of the treating physicians. All patients included in the study are patients of Al-Dalia Medical Center in Hebron West Bank.
Of those 102 patients, all patients (100%) completed the study follow-up (6–12 months), two patients (2%) died during the study period (sudden cardiac death), and seven patients (7%) needed to stop the medication because of the side effects. Three of these patients were exposed to GI problems, two had severe headache and itching, and two suffered from a worsening kidney function.
Prior to starting on LCZ696, all patients were on maximum heart failure therapy, including angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists (ARBs). Once LCZ696 started, it replaced ARBs or ACE inhibitors, with the latter being stopped 36 hours prior to starting LCZ696.
For all patients, demographic data was collected, with the medications and comorbidities listed in the database. During the study, all patient visits to the cardiology clinic were analyzed and tabulated, and hospitalizations and deaths during the study period were recorded. On each visit to the clinic laboratory, results were analyzed. Echocardiograms were performed by multiple operators, and ejection fraction (EF) was calculated per standard protocol. Unfortunately, brain natriuretic peptide (BNP) was not measured for any of the patients because of its unavailability.
Patient follow-up was performed monthly for the first three months and then every three months thereafter.
Measuring quality of life was based on the New York Heart Association classification for heart failure, which was translated to the Arabic language to the best of the treating physician’s ability as described in (Table I).(2)
Table I: Heart failure classification
Class | Patient Symptoms |
I | No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation, or dyspnea (shortness of breath). |
II | Slight limitation of physical activity. Comfortable at rest. Ordinary physical activity results in fatigue, palpitation, or dyspnea (shortness of breath). |
III | Marked limitation of physical activity. Comfortable at rest. Less than ordinary activity causes fatigue, palpitation, or dyspnea. |
IV | Unable to carry on any physical activity without discomfort. Symptoms of heart failure at rest. If any physical activity is undertaken, discomfort increases. |
The data were analyzed using SPSS software version 17 for statistical analysis (Statistical Package for Social Sciences, SPSS Inc., Chicago, Illinois, USA).
Results
Among the 102 patients, 64 were male, 38 were female, and all had different etiologies of reduced ejection fractions. Mean of age was 62 years (see Table II). During the study period, none of the patients needed hospitalization for exacerbation of heart failure. However, two patients (2%) died in the group because of complications of ischemic heart disease and sudden death.
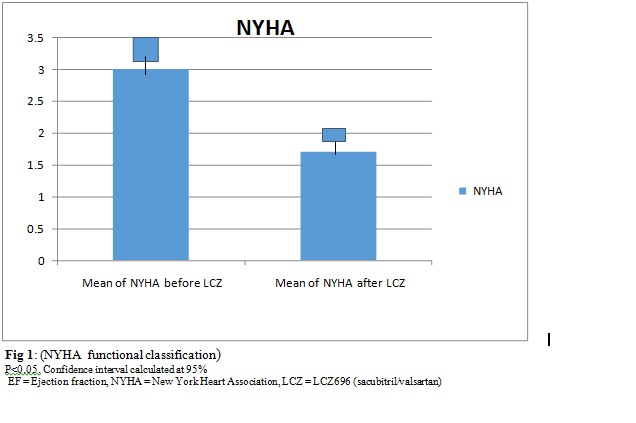
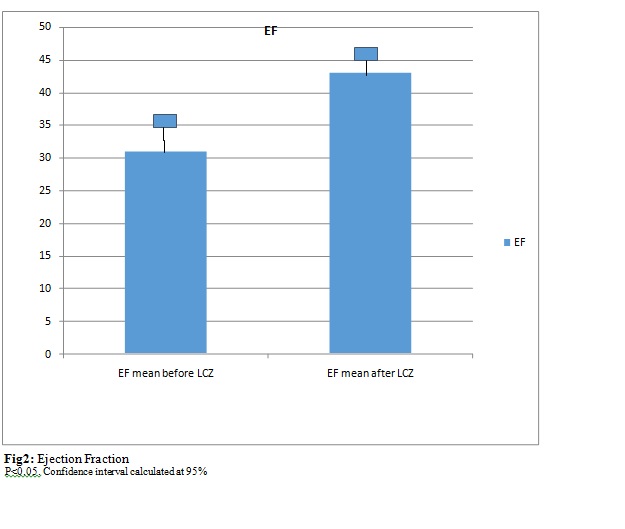
NYHA classification improved by a mean of 1.3 points (about 43% improvements). (Figure1). Ejection fraction (EF) increased by a mean of 12 points (39% improvement) in the study group. See (Figure 2).
Table II (A): Gender
Sex and age of participants |
Male | 64 (63%) |
Female | 38 (37%) |
Total | 102 |
Mean age | 62 |
Table II (B): Medical History
Medicalhistory |
HTN | 58 (57%) |
DM | 41 (40%) |
CHF | 102 (100%) |
Ischemic heart disease | 64 (63%) |
CRTD | 6 (6%) |
AICD | 20 (20%) |
LHC | 68 (67%) |
AF | 21 (21%) |
HTN = hypertension, DM = diabetes mellitus, CHF = congestive heart failure, CRTD = cardiac resynchronization therapy device, AICD = automated implantable cardioverter defibrillator
Table II(C): Medications
Medications of participants |
Diuretics (furosemide) | 90 (88%) |
Beta blockers | 92 (90%) |
Spironolactone | 65 (64%) |
Digoxin | 45 (44%) |
Antiplatelet (aspirin or clopidogrel) | 85 (84%) |
Anticoagulants (Coumadin, apixaban, or rivaroxaban) | 23 (22%) |
Statins | 66 (65%) |
Discussion
Congestive heart failure with reduced left ventricular ejection fraction has been a major health problem worldwide, accounting for a high percentage of hospital admissions and disabilities per year.(4) Despite the standards of care for treatment options, be it the medical options like diuretics, beta blockers, angiotensin-converting enzyme inhibitors (ACE inhibitors), angiotensin II receptor antagonists (ARB), Aldactone, and digoxin or the devices like cardiac resynchronization therapy device (CRTD) and automatic implantable cardioverter-defibrillator (AICD), we still face high mortality to this illness as well as the exhaustion of our health resources. (5,6)
Here in Middle East, particularly in Palestine, the problem of heart failure with reduced EF is not of less importance. On the contrary, it is more apparent and mostly secondary to the high prevalence of coronary artery disease and the delayed treatment of myocardial infarction because of multiple patients and other societal factors. (8) Also, heart failure with reduced EF is caused by the high prevalence of hypertension, dyslipidemia, and uncontrolled diabetes mellitus. (9,10) Increasing salt intake mainly as sodium is also a related factor.(11)
After the introduction of LCZ696 (a combination of ARB and sacubitril, a neprilysin inhibitor) (12) and despite the cost limitation of its widespread use, we noticed patients who received this medication have a noticeable improvement in their quality of life per NHYA, as well as a noticeable increase in EF (never shown before) despite short-term treatment.(1)
Although we practice aggressive lifestyle modifications with all patients with CHF, the magnitude of improvement on the quality of life was much greater after this medication compared with what we are used to. A point worth mentioning is that addiction to salt in the Middle East is enormous.(7,10) Hence, we speculate that this medication, with its intrinsic diuretic effect, has a greater positive effect on our patient population, which is apparent in our study patients who had their required diuretic dose lowered.
In terms of tolerability, the medication was stopped in 7% of the study patients, which is similar to what we noticed in other patients who started on ACE inhibitors or ARBs.(6) Many of the minor side effects were easily managed by adjusting the doses of accompanying medications. None of the patients in the study group needed to stop the medication secondary to hypotension.
We have observed that our results are in agreement to those reported in the PARADIGM HF trial.
Limitations of the Study
•Small number of patients
•Since this was just an observational study, patients paid for this medication as usual. Unfortunately, it is an expensive medication.
•The study was performed in a contained environment where all patients were contacted on regular times and the number of admissions was looked at carefully. Many of the patients might have needed hospitalization but denied admission because of cost.
•Retrospective study (charts review)
•No core lab for echocardiography
•Quality of life assessment was done by physician-based translated NYHA functional classification to the Arabic language.
Conclusion
In this study, despite its limitations, we think that the new medication, LCZ696, has a very promising outcome on EF (first reported) and the quality of life of a group of Palestinian patients with CHFrEF. However, such effect needs to be replicated in other prospective and retrospective studies.
Acknowledgments
we thank Moussa Abu Jbara, MD (National Center of Diabetes and Endocrinology and Genetics; Amman, Jordan), Iyas El-Moussa, MD (Istishari Hospital; Amman, Jordan), and Ayman Hammoudeh, MD (Istishari Hospital; Amman, Jordan) for their review and comments on this manuscript.
References
1. McMurray, J.J., Packer, M., Desai, A.S., Gong, J., Lefkowitz, M.P., Rizkala, A.R., Rouleau, J.L., Shi, V.C., Solomon, S.D., Swedberg, K., and Zile, M.R., 2014. Angiotensin-neprilysin inhibition versus enalapril in heart failure. New England Journal of Medicine, 371(11), pp.993–1004. DOI:10.1056/NEJMoa1409077
2. Adapted from Dolgin, M, Fox, AC, Gorlin, R, Levin, RI, New York Heart Association. Criteria Committee. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Boston, MA: Lippincott Williams and Wilkins; March 1, 1994.
3. Al-Shamiri, MQ. Heart Failure in the Middle East. Current Cardiology Reviews. 2013; 9(2):174–178. DOI: 10.2174/1573403X11309020009.
4. Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart Failure Associated Hospitalizations in the United States. Journal of the American Colleg ofCardiology.2013;61(12):10.1016/j.jacc.2012.12.038. DOI:10.1016/j.jacc.2012.12.038.
5. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: a secondary analysis of the PARADIGM-HF trial by Desai et al. in the JAMA Cardiology journal.
6. Early improvement of symptoms using LCZ696 in a patient with systolic heart failure and a reduced ejection fraction: a case report. Kohlmeier J et al. Perfusion. 2016 Jul 14.
7. McCelvey RS, Yusuf S, Pericak D, et al. Comparison of candesartan, enalapril, and their combination in congestive heart failure: Randomized Evaluation of Strategies for Left Ventricular Dysfunction (RESOLVD) pilot study. Circulation 1999;100:1056–1064
8. Mokdad AH, Jaber S, Aziz MI, et al. The state of health in the Arab world, 1990–2010: an analysis of the burden of diseases, injuries, and risk factors. Lancet 2014; 383:309–20.
9. Juenger J, Schellberg D, Kraemer S, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002; 87:235–241.
10. World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. 2009.
11. Golzarand M, Mirmiran P, Jessri M, et al. Dietary trends in the Middle East and North Africa: an ecological study (1961 to 2007). Public Health Nutrition. 2012; 15:1835–44.
12. Afshin A, Micha R, Khatibzadeh S, On behalf of the 2010 Global Burden of Diseases, Injuries, and Risk Factors Study: NUTRItrition and Chronic Diseases Expert Group (NUTRICODE), and Metabolic Risk Factors of Chronic Diseases Collaborating Group, et al.The impact of dietary habits and metabolic risk factors on cardiovascular and diabetes mortality in countries of the Middle East and North Africa in 2010: a comparative risk assessment analysis. BMJ Open 2015; 5:e006385. DOI: 10.1136/bmjopen-2014-006385
13. Leri A, Liu Y, Li B, et al. Up-regulation of AT1 and AT2 receptors in postinfarcted hypertrophied myocytes and stretch-mediated apoptotic cell death. Am J Pathol. 2000; 156:1663–1672.
14. Dostal DE, Baker KM. Angiotensin II stimulation of left ventricular hypertrophy in adult rat heart: mediation by the AT1 receptor. Am J Hypertens. 1992; 5:276–280.
15. Harrap SB, Dominiczak AF, Fraser R, et al. Plasma angiotensin II, predisposition to hypertension, and left ventricular size in healthy young adults. Circulation 1996; 93:1148–1154.
16. Crabos M, Roth M, Hahn AWA, Erne P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts: coupling to signaling systems and gene expression. J Clin Invest 1994;93:237