ABSTRACT
Introduction: Pilocytic astrocytoma is classified as grade I glioma by the (WHO), and although it remains one of the most frequent oncological pathologies located in posterior fossa, it is an intriguing and unpredictable entity. Pilocytic astrocytomas might be identified supratentorially or in the optic pathway. The close vicinity of these lesions to the brainstem and fourth ventricle zone explains the hazardous presentation of these patients. Large multi-institutional reports illustrating the epidemiology of pilocytic astrocytoma surgery especially in developing countries are lacking.
Objectives: The current research is supplementary to other published works, and it aimed to use a retrospectively collected data registry of surgically treated patients to primarily delineate the relative frequencies of pilocytic astrocytomas. Special emphasis was devoted to analyzing the cardinal epidemiological features and major concurrent issues.
Methods & Patients: This retrospective review data in the Institutional patient database for all patients who underwent surgical intervention in the Department of Neurosurgery at a single referral center – King Hussein Medical Center-over an 18-year period from January 2000 to December 2018 were scrutinized. Clinical records, as well as computed tomography and magnetic resonance imaging (MRI) reports, surgical notes, follow up records , and histopathological reports were obtained from all indexing systems that are used, and the data were analyzed for all patients. Patients within the age range of 1 to 37 years were included in the study.
Results: There were 102-patients in this study; 53 were females and 49 were males (male-to-female ratio of 1:1.1). The mean age was 12.01 years. Topographically, the posterior fossa was the most common region where pilocytic astrocytomas are found, followed by 13 in the supra-sellar region. The most common clinical presentation of pilocytic astrocytoma was a marked increased intracranial pressure. There was cranial nerves involvement in 2.94% of cases. Symptomatic hydrocephalus was evident in 39-patients as the primary presentation. Endocrinological disturbances were manifested in seven Patients. Visual difficulties were identified in six patients. Nine patients showed lesion recurrence and underwent a second surgery and one patient showed a second recurrence with transformation to a grade III tumor. The general complication rate in our study was 21.56 %. Overall, no mortality was correlated to pilocytic astrocytoma.
Conclusions: Pilocytic astrocytoma data from our analysis revealed: a slight female predominance and younger age and presentation in our patients in addition to an insidious clinical demonstration with marked increased intracranial pressure. The largest proportion of pilocytic astocytomas located in the posterior fossa. Treatment options have improved the survival rate, and recurrence rates are very low because of a feasible gross total excision.
Keywords: Pilocytic astrocytoma; grade I; Posterior fossa tumor; Hydrocephalus; Endoscopic third ventriculostomy.
JRMS December 2019;26(3):57-65 :10.12816/0054818
Introduction
Pilocytic astrocytoma is classified as grade I glioma by the World Health Organization (WHO), and although it remains one of the most frequent oncological pathologies located in posterior fossa, it is an intriguing and unpredictable entity. Progression to higher grade astrocytomas and dissemination are extremely rare. Pilocytic astrocytomas might be identified supratentorially or in the optic pathway, but the posterior fossa is the most common location [1-5]. The close vicinity of these lesions to the brainstem and fourth ventricle zone explains the hazardous presentation of these patients with markedly increased intracranial pressure, obstructive hydrocephalus and cranial nerves involvement as described in about 80% of the patients, which leads to herniation and death [Figure. 1].
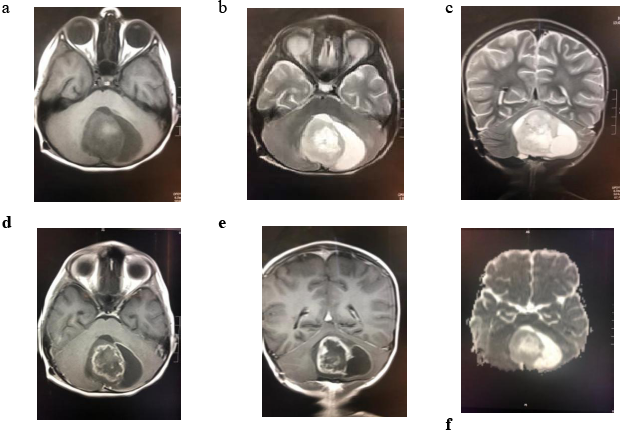
Figure 1: radiological Images show; (a) axial T1WI ,(b)axial T2WI,(c) coronal T2WI shows characteristic cerebellar cyst with large mural nodule compressing and effacing the 4th ventricle and the brain stem with no significant surrounding vasogenic edema,(d) and (e) axial and coronal T1 post contrast shows heterogenous intense peripheral enhancement within the solid component with rim wall enhancement within the cystic component, (f) ADC map showing no restriction in diffusion to differentiate from other posterior fossa tumors as medulloblastoma.
More than 60% of tumors arising in children are in the posterior fossa [4, 6-8]. Pilocytic astrocytoma shows low mitotic activity and very low malignant transformation potential [9]. Innovative progressions in radiological modalities (apparent diffusion coefficient (ADC)) and treatment options have shown a great improvement in survival in these patients over the past few decades [4, 10-14]. Large multi-institutional reports illustrating the epidemiology of pilocytic astrocytoma surgery especially in developing countries are lacking [15-17].
The current research is supplementary to other published works, and it aimed to use a retrospectively collected data registry of surgically treated patients to primarily delineate the relative frequencies of pilocytic astrocytomas. Special emphasis was devoted to analyzing the cardinal epidemiological features and major concurrent issues.
Methods & Patients
This retrospective review was conducted with the approval of the institutional ethics committee. Data in the Institutional patient database for all patients who underwent surgical intervention in the Department of Neurosurgery at a single referral center – King Hussein Medical Center-over an 18-year period from January 2000 to December 2018 were scrutinized. Clinical records, as well as computed tomography and magnetic resonance imaging (MRI) reports, surgical notes, follow up records, and histopathological reports were obtained from all indexing systems that are used, and the data were analyzed for all patients. Patients within the age range of 1 to 37 years were included in the study.
Results
There were 102 patients enrolled in this study after applying the inclusion criteria were. Among these patients 53 were females and 49 were males (male-to-female ratio of 1:1.1). The mean age was 12.01 years(range, 1-37-years). Topographically, the posterior fossa was the most common region where pilocytic astrocytomas are found, followed by 13 in the supra-sellar region [Figure.2], and 12 in different lobes of the brain [Figure.3].
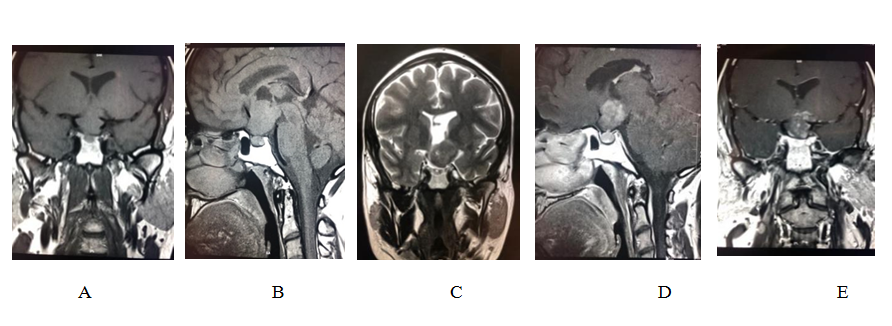
Figure 2: Radiological Images of a chiasmatic lesion identified in 35-yr old male patients; (a )& (b) Pituitary protocol small field of view T1WI coronal and sagittal MRI demonstrate a fairly defined isointense suprasellar lesion involving the optic chiasm and pushing the pituitary stalk to the right, the lesion is extending superiorly to the third ventricle, (c ) coronal brain T2WI shows heterogenous intensity of the lesion with solid and cystic components. (d)& (e) pituitary protocol T1WI post contrast in coronal and sagittal reformats demonstrate heterogenous enhancement within the solid components.
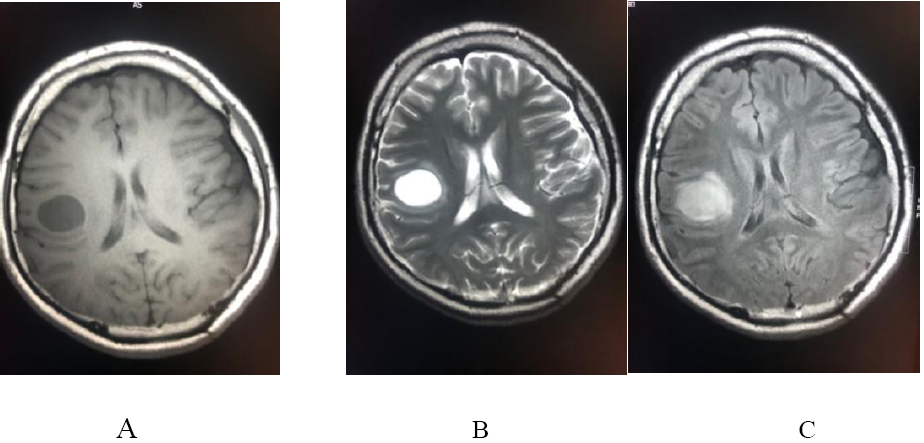
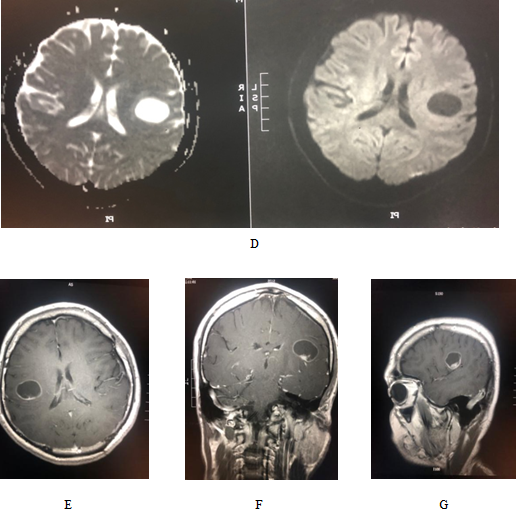

Figure 3: Showing the radiological images of a 28-yr old male patient; (a) & (b) axial T1 &T2WI : well defined rounded right parietal lesion, appears hypointense in T1 and hyperintense in T2 with no significant mass effect on adjacent cortex nor vasogenic edema, (c) axial FLAIR : the lesion appears heterogeneously hyperintense mainly cystic with solid component posteroinferiorly, (d) diffusion and ADC map : no restricted diffusion( in contrast to abscess ) . (e), (f) & (g): axial ,coronal and sagittal post contrast : the lesion exhibits avid enhancement in the mural nodule with thin regular ring wall enhancement.
Six patients had thalamic pilocytic astrocytomas, four patients had intraventricular tumors and two patients had spinal tumors. The most common clinical presentation of pilocytic astrocytoma was a marked increased intracranial pressure. Headache and vomiting were the most common presenting symptoms with the duration of symptoms ranging from 1- 12-months in extreme cases. There was cranial nerves involvement in 2.94% of cases. All patients underwent (MRI) that included all the required sequences.
Symptomatic hydrocephalus was evident in 39-patients as the primary presentation, and a pre-operative ventriculo-peritoneal shunt inserted was in 17patients, while 22-patients underwent endoscopic third ventriculostomy (ETV), and two patients needed post-tumor resection shunt procedures. Endocrinological disturbances were manifested in seven Patients. Visual difficulties were identified in six patients. All patients underwent surgical intervention via a craniotomy/craniectomy with total excision of the tumoral nodule in addition to the cystic part. Nine patients showed lesion recurrence and underwent a second surgery and one patient showed a second recurrence with transformation to a grade III tumor. The general complication rate in our study was 21.56 %, and 22-patients were affected as follows: nine-patients developed recurrence, and four- patients developed permanent endocrinological conditions, three patients developed a visual deficit, four patients developed mutism and two patients developed ataxic gait. Overall, no mortality was correlated to pilocytic astrocytoma.
Discussion
Pilocytic astrocytoma is considered to be benign and slow-growing lesion, which seldom spreads to adjacent structures or undergoes malignant transformation [9]. Pilocytic astrocytoma isconsidered to be a challenge because all patients experience the same type of vexes as other posterior fossa lesions. The most recent histopathological classification (2016) includes a combination of both histological and genetics conclusions. Pilocytic astrocytoma is categorized as a grade I tumor that most frequently occurs in the cerebellum[18, 19]. However, the supra-sellar, optic pathway, spinal, intraventricular and supra-tentorialpilocytic astrocytoma is an uncommon but well-known entity [19-21]. Spinal cord pilocytic astrocytoma comprises only about 2–5.2% of all pilocytic astrocytomas, while it accounts for 12.4% of all primary spinal cord tumors in children /adolescents and 0.8% in adults [19, 22-24]. Our research showed that 1.96% were spinal pilocytic astrocytomas [Figure. 4].
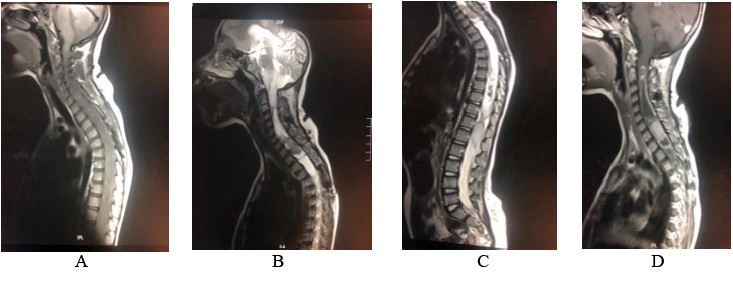
Figure 4: Radiological study for 12-yr of female presented with mylopathic features; (a ) sagittal cervicothoracic T1WI: Extensive expansion with heterogenousiso/hypointense mass along the spinal cord. (B) & (c) sagittal cervicothoracic and whole spine T2WI: large diffuse mixed solid/cystic intramedullary mass lesion extending along the whole cord from the medulla oblongata ending opposite L1/2. No vertebral destruction, the lesion is causing expansion of the spinal canal. (D) the lesion exhibit strong heterogenous enhancement in the solid components.
Histologic examination typically has a known biphasic pattern that is composed of varying portions of compact piloid areas that alternate with spongy loosely textured areas, which consist of multipolar cells, microcytes, and eosinophilic granular bodies, as well as densely fibrillated tapered corkscrew shaped, brightly eosinophilic hyaline figures (Rosenthal fibres), also calcifications [19, 25]. Tumor cells have immunoreactivity for glial fibrillary acidic protein (GFAP), while Ki67 immunostaining shows a low proliferation index [Figure. 5].
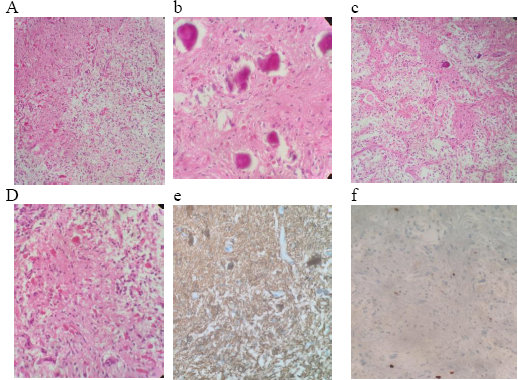
Figure 5: Histopathological images revealed: (a)biphasic pattern composed of varying portion of compact piloid areas alternating with spongy loosely textured areas containing microcytes, (b)biphasic pattern and calcification (c) Areas of calcifications and Rosenthal fibers (d) densely fibrillated tapered corkscrew shaped, brightly eosinophilic hyaline figures (Rosenthal fibers), also calcifications (e)Tumor cells have immunoreactivity for glial fibrillary acidic protein (GFAP), (f) Ki67 immunostaining shows a low proliferation index.
The clinical representation is insidious and depends on the site of the tumor, biological behavior and the rate of growth. Clinical manifestations differ from non-definite symptoms such as headache and vomiting to more intense and serious presentations such as lower cranial nerves palsies, visual deficits, endocrinological syndromes, and ataxia resulting from direct involvement or compression [1, 4, 5]. In this review, the most frequent clinical presentation is the marked increase in intracranial pressure, headache and vomiting, followed by cerebellar symptoms, visual disturbances and endocrinological manifestations.
The value of radiological imaging to make a diagnosis based on medical history and physical examination results is of the utmost importance. All patients underwent a magnetic resonance imaging (MRI), and specific relevant studies (apparent diffusion coefficient) as required. Classical MRI findings of pilocytic astrocytoma are a cystic mass with a mural nodule; the solid component is hypo-intense on T1WI and hyper-intense on T2WI. Several studies established that most pilocytic astrocytoma cyst walls do not enhance while some may be enhanced intensely [26]. In our study these radiological studies were sufficient and appropriate in all patients to consider and plan the surgical management [Figure. 6].
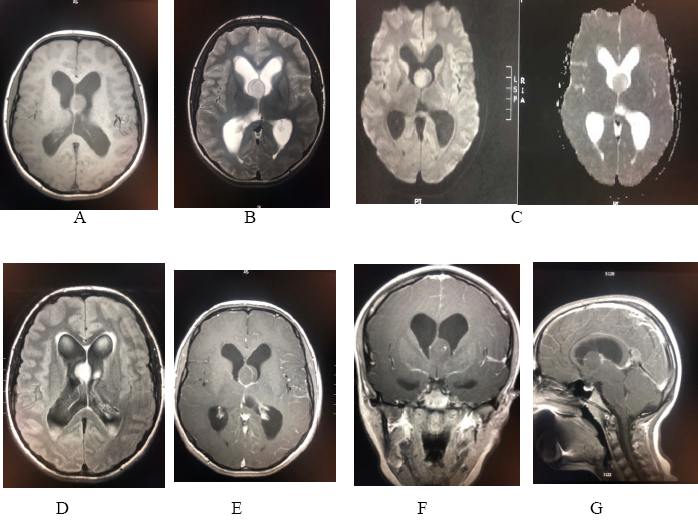
Figure 6: Radiological images of a 16-years female presented with marked raised intracranial pressure revealed: (a) & (b) axial T1WI & T2WI a well-defined rounded T1 isointense/ T2 hyperintense intraventricular lesion attached to the septum pellucidum .The lesion appears homogenous ( in contrast to central neurocytoma). (c) Diffusion and ADC map study : no restriction diffusion. (d) axial FLAIR : the lesions appears hyperintense with associated hydrocephalus changes with transependymal edema, the location is far from foramen of Monro ( to differentiate from colloid cyst). (e) (f) (g) axial, coronal and sagittal T1 post contrast : the lesion exhibit mild spotted enhancement in the center.
Because there is limited space in the posterior compartment, all lesions were addressed utilizing surgical intervention, which was a posterior craniectomy with excision of the tumor performed on an urgent basis. Treating the associated hydrocephalus in these patients is the major issue. Other zones lesions were treated expectantly. The nature and severity of the deficits show a discrepancy between patients with both the patient and treatment dynamics contributing to the ultimate outcomes.
In our management armamentarium the surgical therapy is a corner stone in the treatment options for pilocytic astrocytoma patients to achieve better results and higher survival rates, and we followed the most recent guidelines.
Conclusion
Pilocytic astrocytoma data from our analysis was comparable to the data from a review of the Surveillance, Epidemiology, and End Results (SEER) database, with a slight female predominance and younger age and presentation in our patients in addition to an insidious clinical demonstration with marked increased intracranial pressure. The largest proportion of pilocytic astocytomas located in the posterior fossa. Innovative evolutions in radiological modalities and treatment options have improved the survival rate, and recurrence rates are very low because of a feasible gross total excision.
Conflict of Interest Statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding: Authors disclose no external funding sources.
References
1. Kristiansen I, Strinnholm M, Stromberg B, Frisk P. Clinical characteristics, long-term complications and health-related quality of life (HRQoL) in children and young adults treated for low-grade astrocytoma in the posterior fossa in childhood. Journal of neuro-oncology. 2019;142(1):203-10.
2. Buus-Gehrig C, Lehrnbecher T, Porto L, Becker M, Freiman T, Mittelbronn M, et al. Pontine tumor in a neonate: case report and analysis of the current literature. Journal of neurosurgery Pediatrics. 2019:1-7.
3. Choque-Velasquez J, Hernesniemi J. Unedited microneurosurgery of a posterior fossa pilocytic astrocytoma. Surgical neurology international. 2018;9:235.
4. Al Rawabdeh S, Alqroom R, Al-Khawaldeh M, et al. Posterior fossa tumors: Pandora's Box violated. IJSR.2019; 8(1): 480-82.
5. Tu A, Robison A, Melamed E, Buchanan I, Hariri O, Babu H, et al. Proliferative Index in Pediatric Pilocytic Astrocytoma by Region of Origin and Prediction of Clinical Behavior. Pediatric neurosurgery. 2018;53(6):395-400.
6. Ostrom QT, de Blank PM, Kruchko C, et al. Alex's Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro-oncology. 2015; 16 Suppl 10:1-36.
7. Kushel YV, Sorokin VS, Chel'diev BZ, Tekoev AR. Surgery of posterior cranial fossa tumors in children in the prone position. The surgical technique features. Zhurnal voprosy neirokhirurgii imeni N N Burdenko. 2018;82(3):36-41.
8. Gudrunardottir T, Morgan AT, Lux AL, Walker DA, Walsh KS, Wells EM, et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: The Iceland delphi results. Childs Nerv Syst. 2016;32:1195–203.
9. Wang W, Cheng J, Zhang Y, Wang C. Use of Apparent Diffusion Coefficient Histogram in Differentiating Between Medulloblastoma and Pilocytic Astrocytoma in Children. Medical science monitor: international medical journal of experimental and clinical research. 2018;24:6107-12.
10. Alqroom R, Khasawneh N, Haddad F, Alqurashi M. Neuroendoscopy Sway in the Treatment of Posterior Fossa Tumours associated Hydrocephalus in Children. J Neurol Stroke. 2017; 6(3): 00203.
11. Bognar L, Borgulya G, Benke P, et al. Analysis of CSF shunting procedures requirement in children with posterior fossa tumors. Childs Nerv Syst.2003; 19:332–336.
12. Bohm B, Mohadjer M, Hemmer R. Preoperative continuous measurements of ventricular pressure in hydrocephalus occlusus with tumors of the posterior fossa: the value of ventriculoatrial shunt. Adv Neurosurg. 1978; 5:194–198.
13. Lee M, Wisoff JH, Abbott R, et al. Management of hydrocephalus in children with medulloblastomas: prognostic factors for shunting. Pediatr Neurosurg. 1994; 20:240–247.
14. Bonfield CM, Steinbok P. Pediatric cerebellar astrocytoma: A review. Childs Nerv Syst. 2015;31:1677–85.
15. Alqroom R, Malabeh Q, Makhamreh B.et al. Medulloblastoma: Recapitulate divergent tumoral cells. IJSR.2019; 8(2): 1-3.
16. Packer RJ. Childhood brain tumors: Accomplishments and ongoing challenges. J Child Neurol. 2008;23:1122–7.
17. Totapally BR, Shah AH, Niazi T. Epidemiology and short-term surgical outcomes of children presenting with cerebellar tumors. Clinical neurology and neurosurgery. 2018;168:97-101.
18. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131: 803-820.
19. She DJ, Lu YP, Xiong J, Geng DY, Yin B. MR imaging features of spinal pilocytic astrocytoma. BMC medical imaging. 2019;19(1):5.
20. Motoyama HL, Yamada S, Nakada S, Kurose N, Tanimoto A. Intraorbital ancient pilocytic astrocytoma of the optic nerve in neurofibromatosis type 1 patient presenting with sudden ocular pain. SAGE open medical case reports. 2018;6:2050313x18761310.
21.Burger PC, Scheithauer BW. Pilocytic astrocytoma. In: Font RL, Croxatto JO, Rao NA, editors. (eds) Tumors of the central nervous system (atlas of tumor pathology). Washington, DC: Armed Forces Institute of Pathology, 2007, pp. 89–106.
22. Burkhard C, Di Patre PL, Schuler D, Schuler G, Yasargil MG, Yonekawa Y, Lutolf UM, Kleihues P, Ohgaki H. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg. 2003;98(6):1170–1174.
23. Johnson DR, Brown PD, Galanis E, Hammack JE. Pilocytic astrocytoma survival in adults: analysis of the surveillance, epidemiology, and end results program of the National Cancer Institute. J Neuro-Oncol. 2012;108(1):187–193.
24. Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro-Oncology. 2017;19(suppl_5):v1–v88.
25. Minehan KJ, Brown PD, Scheithauer BW, Krauss WE, Wright MP. Prognosis and treatment of spinal cord astrocytoma. Int J Radiat Oncol Biol Phys. 2009;73(3):727–733.
26. Kobayashi K, Imagama S, Kato F, Kanemura T, Sato K, Kamiya M, Ando K, Ito K, Tsushima M, Matsumoto A, et al. MRI characteristics of spinal ependymoma in WHO grade II: a review of 59 cases. Spine (Phila Pa 1976). 2017.